Medical Devices and Medical Supplies Policy
12/30/2025

This policy outlines the requirements you may need to meet in order to sell Medical Devices and Medical Supplies on TikTok Shop.Key Points:
- To sell Medical Devices and Medical Supplies, you may be required to submit documentation for review through the Qualification Center. Requirements vary depending on whether you are the product’s manufacturer, importer, repacker, or reseller.
- To better understand the category review process and how to respond to rejections, refer to Your Guide to Category Qualification.
Medical Devices and Medical Supplies
Medical devices are regulated by the U.S. Food and Drug Administration (FDA). They are also classified based on their intended use. Click here to check if your product is a medical device and view its classification.The categories include products that are considered medical devices. The list is not limited to these examples.
- Baby & Maternity
- Pacifiers, teethers, teething relief, and dental care
- Ear and nose care
- Sunglasses
- Breast pumps and accessories
- These products require additional documentation. For more information, see Baby and Maternity Products Policy.
- Beauty & Personal Care
- Ice packs
- Heat patches
- Facial beauty devices
- Hair removal devices
- Earwax removal tools
- Massage tools
- Toothbrushes
- Teeth whitening devices
- Denture care
- Dental floss and picks
- Tampons
- Menstrual cups and pads
- Sanitary tools
- Reading glasses
- Glasses Cases
- Contact lens applicators
- Fashion Accessories
- Sunglasses
- Replacement lenses
- Non-prescription glasses
- Sports & Outdoor
- Hiking sticks
- Walking poles
- Cycling and fishing glasses
- Sunglasses
- Golf and skiing sunglasses
- Sports eyewear
- Sports sleeves and support
- First aid kits
- Health
- Thermometers
- Pressure monitors
- Home test kits
- Medical and PPE masks (including respiratory masks)
- Ointments and antiseptics
- Bandages, dressings, and liquid bandages
- Heating pads, heating patches, and heating wraps
- Saline sprays
- Nicotine replacement products
- Gums
- Lozenges
- Patches
- Inhalers
- Wheelchairs
- The following health medical devices can be offered by invite-only sellers:
- Glucose Monitors
- Electric/manual blood pressure monitors
- Cholesterol monitors
- Glucose control solutions
- Glucose kits
- These products are only available to invite-only sellers and have additional requirements/best practices. See Promoting Sexual Wellness Products for more information.
- Fertility thermometers
- Ovulation and fertility test kits
- Pregnancy tests
- Male and female fertility tests
- Male and female condoms
- Lubricants
Prohibited Products
The following products are not permitted on TikTok Shop:- Class III medical devices
- Products that require a prescription issued by a licensed healthcare provider
Requirements To Sell Medical Devices and Medical Supplies Class I and II
You may be required to pass category qualification to sell Medical Devices and Medical Supplies. This process requires submitting documents proving you are qualified to sell these products.Detailed requirements are listed in the sections below. Open each section to view what is needed.
Manufacturers, Importers, And Repackers
If you are applying to sell Medical Devices and Medical Supplies Class I and II you manufactured, imported, or repacked, you are required to submit the following documents:
1. Proof of FDA Establishment Registration & Device Listing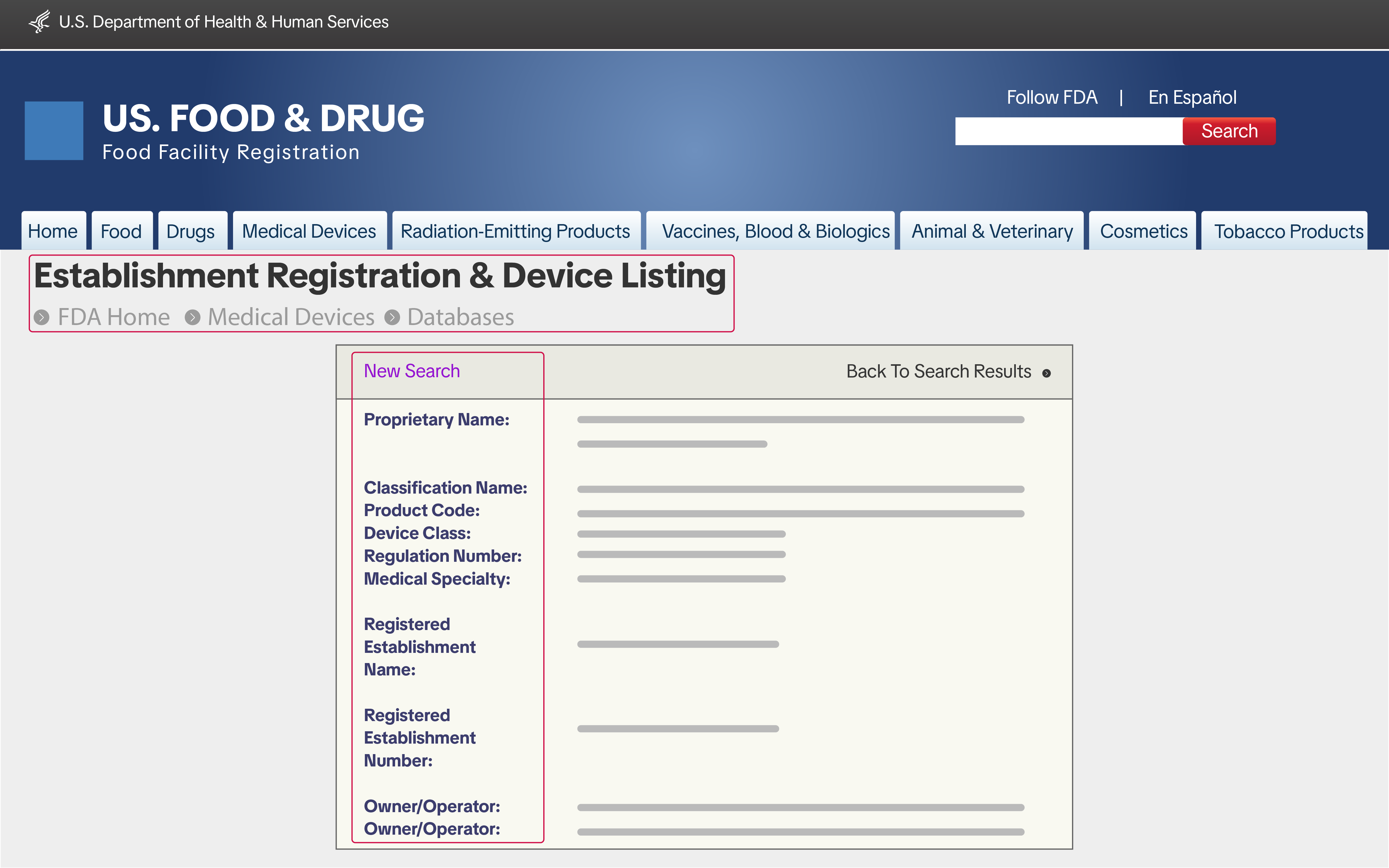 Manufacturers, importers, and repackers are required to submit proof of FDA Establishment Registration and proof of Device Listing. You can get this information from the FDA's Unified Registration and Listing System (FURLS). The following documents must be provided:
Manufacturers, importers, and repackers are required to submit proof of FDA Establishment Registration and proof of Device Listing. You can get this information from the FDA's Unified Registration and Listing System (FURLS). The following documents must be provided:
 Class I Medical DevicesManufacturers, importers, and repackers must provide images of the product and its packaging.
Class I Medical DevicesManufacturers, importers, and repackers must provide images of the product and its packaging.
The images must meet the following requirements:
1. Proof of FDA Establishment Registration & Device Listing

- A screenshot of the FDA Establishment Registration number (21 CFR Part 807) from the FURLS
- A screenshot of the FDA Device Listing (21 CFR Part 807) from FURLS
- The name and physical address of the registered establishment
- The operation type of the establishment
- Confirmation that the registration is valid at the time of submission
- Screenshots must show that they were taken from the official FDA FURLS website
- All documents must be authentic and remain in their original (unmodified) format
- FDA 510(k) Premarket Notification approval letter (21 CFR 807 Subpart E), or
- Proof of 510(k) exemption from the FDA’s Product Classification database
- The name of the manufacturer or importer
- The product name and category that matches the listing on TikTok Shop. This includes the product description and details
- All documents must be authentic and remain in their original (unmodified) format

The images must meet the following requirements:
- Show all sides of the product and packaging
- Be clear, legible, complete, unedited, and in color
- Have a minimum resolution of 600 × 600 pixels
- Avoid black-and-white, angled, blurry, cropped, or edited images
- Ensure all information on the product and packaging is visible and legible. This includes product description, warning logos, and any other required information
- Brand name
- Device name
- Name and place of business of the manufacturer, packer, importer, distributor, or authorized representative, including street address, city, state, and postal code
- Compliance markings
- Intended use
- Images are clear and legible
- Images reflect the product listed on related documents. This may include FDA documentation or invoices
- Product instructions, manuals, safety information, labels, and warnings are provided in the language of the intended country of sale
- If the product is a set or bundle, include images of each individual item
Resellers
If you are applying to sell Medical Devices and Medical Supplies Class I and II as a reseller, you may be required to submit the following documents:
1. Purchase Invoice or Proof of Purchase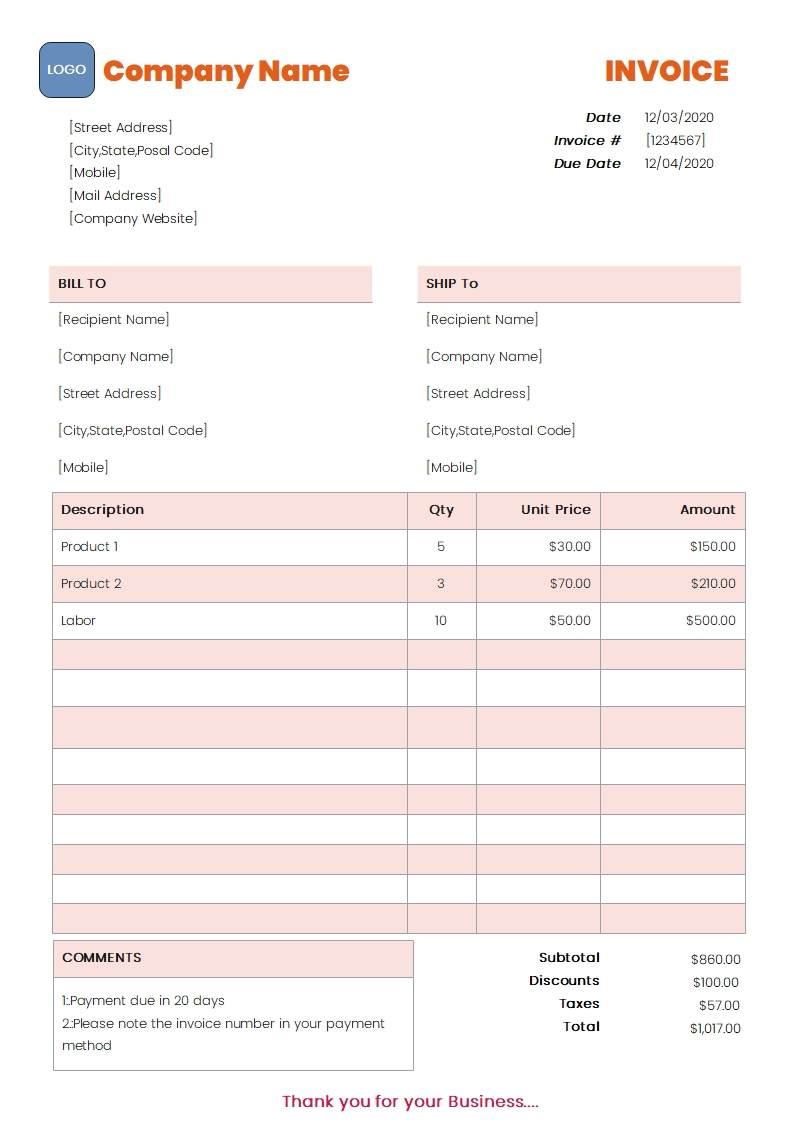 Resellers must submit a legible purchase invoice or proof of purchase issued by the product’s manufacturer or distributor. The invoice must meet all of the following requirements:
Resellers must submit a legible purchase invoice or proof of purchase issued by the product’s manufacturer or distributor. The invoice must meet all of the following requirements:
 Class I Medical DevicesResellers must provide images of the product and its packaging.
Class I Medical DevicesResellers must provide images of the product and its packaging.
The images must meet the following requirements:
1. Purchase Invoice or Proof of Purchase

- Be dated within the last 180 days
- Show the same name and address as the selling account
- Include the full name and address of the manufacturer or distributor
- Contain products belonging to the applicable category
- Reflect a combined purchase of at least 400 units
- Be written in English or Chinese
- Pricing information may be omitted (optional)
- TikTok Shop reserves the right to verify the submitted documentation by contacting the product vendor listed on the invoice
- Retail order confirmations or invoices are not accepted

The images must meet the following requirements:
- Show all sides of the product and packaging
- Be clear, legible, complete, unedited, and in color
- Have a minimum resolution of 600 × 600 pixels
- Avoid black-and-white, angled, blurry, cropped, or edited images
- Ensure all information on the product and packaging is visible and legible, including product description, warning logos, and any other required information
- Brand name
- Device name
- Name and place of business of the manufacturer, packer, importer, distributor, or authorized representative, including street address, city, state, and postal code
- Compliance markings
- Intended use
- Images are clear and legible
- Images reflect the product listed on related documents. This may include FDA documentation or invoices
- Product instructions, manuals, safety information, labels, and warnings are provided in the language of the intended country of sale
- If the product is a set or bundle, include images of each individual item
Additional Requirements
These requirements are in addition to the documentation required under your seller type above.Open each section below to see the specific requirements for different types of medical devices.
Electrical Medical Devices


For Manufacturers or Importers:
- A valid Certification of Compliance/Conformity (COC) accredited by OSHA. The COC must:
- Include a clear description and details of the tested product
- Match the product being listed on TikTok Shop
- List all safety standard tests performed with Pass/Fail indicators
- Be issued within the past 2 years
- Include the name and address of the accredited laboratory
- Correspond to the product photos submitted
- Image of a valid Product Electrical Safety Marking
- Image of a valid Product FCC Safety Marking (if the product has Bluetooth or Wi-Fi)
For Resellers:
- Image of a valid Product Electrical Safety Marking
- Image of a valid Product FCC Safety Marking (if the product has Bluetooth or Wi-Fi)
Surgical N95 Respirators
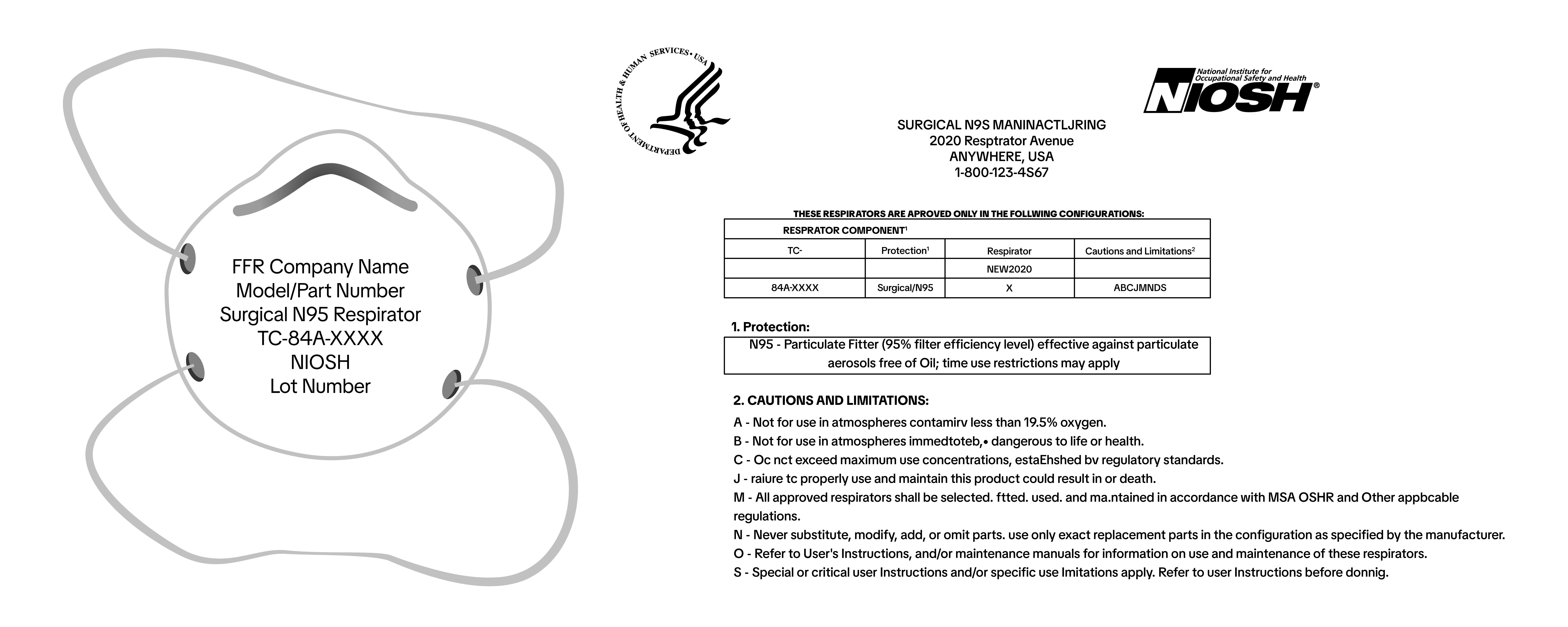
- Product Label on Mask:
- Approval Holder / Company name
- Model or Part number
- “Surgical N95 Respirator” text
- TC-Approval Number
- NIOSH (in capital block letters)
- Lot number
- Packaging Label:
- Expiration date / Use by date
- Packaging Label and/or Insert:
- Full NIOSH surgical N95 respirator approval label, often located on or within the packaging
PPE Masks (Medical masks with liquid barrier protection)

- Packaging Label:
- ASTM F2100 or EN 14683 standard on the box label
- Expiration date / Use by date
Ear Drops, Eye Drops, Contact Lens Solutions, and Contact Lens Conditioning Kits
Due to safety and compliance considerations, only select qualified sellers are permitted to offer these products on TikTok Shop. In addition to the standard documentation required for your seller type, qualified sellers must submit:
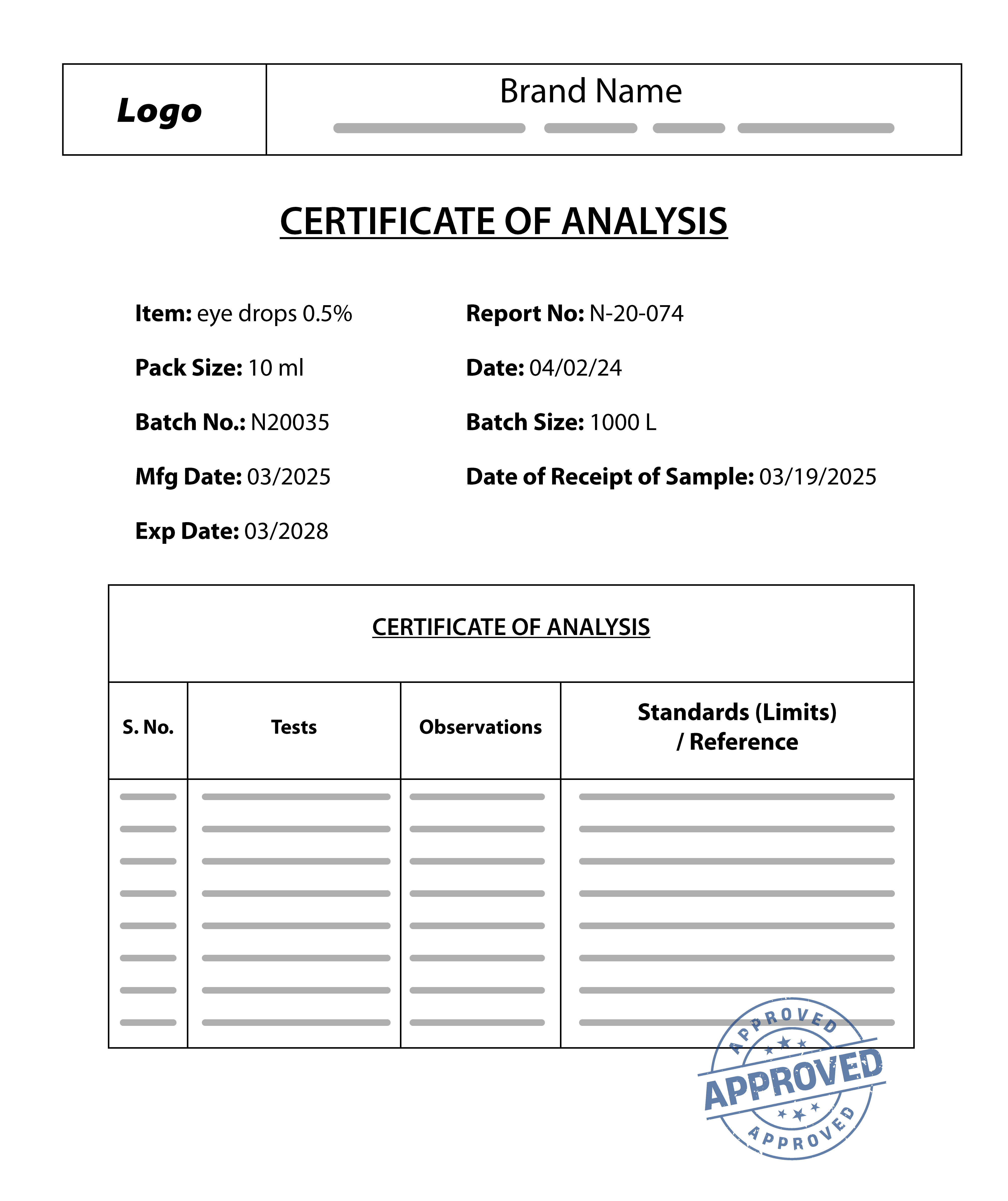 The COA verifies product quality, safety, and regulatory compliance. It must:
The COA verifies product quality, safety, and regulatory compliance. It must:
- Certificate of Analysis (COA)

- Contain a clear description and relevant details of the tested product
- Be issued within the past 2 years
- Match the product being listed
- List all safety standards tested
- Include the name and address of the accredited testing laboratory
- Be provided in English
- Be clear, unaltered, and legible
Over the Counter (OTC) Medication Requirements (Invite Only)
This section details the requirements that invite-only sellers must follow when selling Over-the-Counter (OTC) medications on TikTok Shop. Sellers who are not invited to sell OTC medication are prohibited from selling them.
The following products may be offered by invited sellers: Manufacturers, importers, and repackers must provide a General Certificate of Conformity (GCC) that includes:
Manufacturers, importers, and repackers must provide a General Certificate of Conformity (GCC) that includes:
Accepted Test Report TypesThe test report must be one of the following:
 Resellers must submit a legible purchase invoice or proof of purchase issued by the product’s manufacturer or distributor. The invoice must meet all of the following requirements:
Resellers must submit a legible purchase invoice or proof of purchase issued by the product’s manufacturer or distributor. The invoice must meet all of the following requirements:
The following products may be offered by invited sellers:
- Antifungals
- Yeast infection treatments
- Thrush treatments
- Acne treatments
- Nasal treatments
- Nail fungus treatments
- Jock itch treatments
- Dandruff treatments
- Athlete’s foot treatments
- Scars & Stretchmarks
- Stretchmark removal creams, gels, lotions, and oils
- Scar tapes or patches
- Allergies, Sinus & Asthma
- Allergy relief products
- Sinus congestion treatments
- Asthma management products
- Pain Relief
- Acetaminophen and ibuprofen
- Muscle stimulators and accessories
- Joint or muscle pain relief rubs or medications
- Homeopathic remedies
- Eczema, Psoriasis & Rosacea Care
- Topical creams or treatments for eczema, psoriasis, or rosacea
- Cuts & Wounds
- Products that support healing for cuts and wounds
- Digestion & Nausea
- Products that relieve digestive discomfort or nausea
- Coughs & Colds
- Products that relieve cough or cold symptoms
Manufacturers, Importers, and Repackers
- General Certificate of Conformity (GCC)

- Product description: A clear and specific description of the product covered by the GCC.
- Applicable product safety rules: List all relevant safety rules, including:
- Compliance with 21 CFR Part 73 Subpart B – Drugs (approved color additives permitted in OTC medications)
- Testing details: Date(s) and location where the product was tested for each consumer product safety rule listed.
- Certifying party information: Name, full mailing address, and telephone number of the U.S. domestic manufacturer or importer certifying the product.
- Record keeper information: Name, full mailing address, email address, and telephone number of the person responsible for maintaining test records.
- Manufacturing details:
- Month and year of manufacture
- Country, and if applicable, state and city/administrative region where the product was manufactured or assembled
- If multiple facilities are in the same city, include the specific street address of the manufacturing location
- Product Lab Test Report
Accepted Test Report TypesThe test report must be one of the following:
- A valid test report from TikTok Shop Accredited Partner Laboratories (listed below); or
- A valid test report from an in-house ISO/IEC 17025–accredited laboratory, plus a valid ISO certificate; or
- A valid test report from an in-house laboratory, plus a valid cGMP certificate compliant with 21 CFR 111 or 21 CFR 117.
- Eurofins
- NSF (National Sanitation Foundation) International
- SGS (General Society of Surveillance)
- UL (Underwriters Laboratories) Solutions
- TÜV Sud
- Mérieux NutriSciences
- Intertek
- Certified Laboratories
- Issued within the last 180 days
- Cover products that match the purchase invoice, including brand and manufacturer details
- Display the same company name and address as the selling account
- If the seller is the manufacturer, the manufacturer listed on the report must match the selling account
- Microbiological testing
- Heavy metals testing
- Contaminant testing, such as pesticides or residual solvents (as labeled on the report)
- Content claims / potency / label claim verification
- The reported amount of each active ingredient per dosage unit must fall within the permitted tolerance range
Resellers
- Purchase Invoice or Proof of Purchase

- Be dated within the last 180 days
- Show the same name and address as the selling account
- Include the full name and address of the manufacturer or distributor
- Contain products belonging to the applicable category
- Reflect a combined purchase of at least 400 units
- Be written in English or Chinese
- Pricing information may be omitted (optional)
- TikTok Shop reserves the right to verify the submitted documentation by contacting the product vendor listed on the invoice
- Retail order confirmations or invoices are not accepted
- Product Lab Test Report
- Eurofins
- NSF (National Sanitation Foundation) International
- SGS (General Society of Surveillance)
- UL (Underwriters Laboratories) Solutions
- TÜV Sud
- Mérieux NutriSciences
- Intertek
- Certified Laboratories
- Issued within the last 180 days
- Cover products that match the purchase invoice, including brand and manufacturer details
- Display the same company name and address as the selling account
- If the seller is the manufacturer, the manufacturer listed on the report must match the selling account
- Microbiological testing
- Heavy metals testing
- Contaminant testing, such as pesticides or residual solvents (as labeled on the report)
- Content claims / potency / label claim verification
- The reported amount of each active ingredient per dosage unit must fall within the permitted tolerance range
Enforcement Actions and Appeals
Claiming electrical safety without approved testing or certification violates this policy. Tests and certifications must come from recognized electrical safety or FCC laboratories. Products are also in violation if they have inaccurate or fraudulent labeling.We regularly review your shop’s compliance with this policy. If any violations are identified, TikTok Shop may take enforcement action at our sole discretion. This may include, but is not limited to:
- Rejecting your category qualification application
- Removing your ability to sell in this category
- Deducting points from your account health
- Removing product listings
How to Submit Your Documentation
For categories under category qualification, submit your documentation via the Qualification Center in Seller Center. Click here or follow these steps:- Log into your Seller Center account.
- Click your shop icon in the top right corner.
- Go to My Account > Account Settings.
- Select Qualification Center.
- Click on Category Qualification.
- Click Add Category Authorization and follow the prompts to submit your application.
Product-Specific Documentation
SubmissionSome products require additional documentation when being submitted through Seller Center. This includes certain OTC products. To submit product-specific documentation:
- Log into Seller Center.
- Select Products > Add Products.
- Select Add product. Begin entering your product information, category, images, description, details, etc.
- Fill out the Product Compliance section and submit your documents.
- Once all information has been added (including shipping information), select Submit for review.
How To Address A Category Qualification Rejection
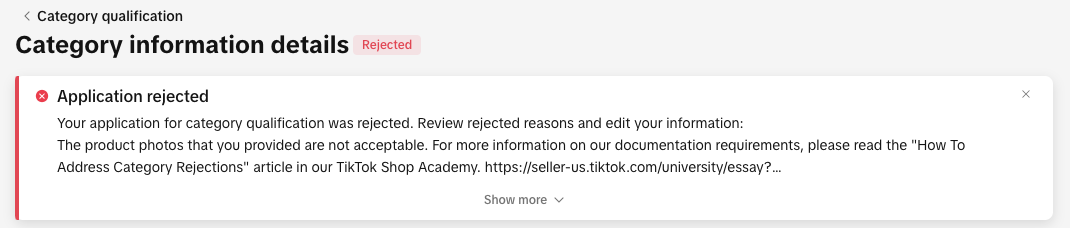
You can view your rejection message by:
- Clicking the bell icon at the top of your Seller Center homepage to go to your inbox
- Opening the rejected application in the Qualification Center.
- Go to the Qualification Center, then click Category Qualification.
- Click the rejected category to view the rejection reason.
- Review the related category policy to confirm what’s needed
- Update or replace your documentation to meet the category’s requirements.
- Click Resubmit to submit your revised application.