Baby and Maternity Products Policy
01/12/2026

This policy outlines the requirements for selling Baby and Maternity Products on TikTok Shop.Key Points:
- To sell Baby and Maternity Products, you may need to submit documentation through the Qualification Center. Requirements vary depending on your role as a seller.
- Ensure you are also complying with the Consumer Product Safety Commission (CPSC). Use a choking hazard warning label if it meets the CPSC’s criteria for small parts.
- For help with the review process and rejection handling, see Your Guide to Category Qualification.
Baby and Maternity Products
The following Baby and Maternity Products are allowed to be sold on our platform through category qualification. This list is non-exhaustive.- Baby and Maternity Products (Typical)
- Baby clothing
- Baby toys and books
- Playmats
- Baby brushes and combs
- Baby bottles and utensils
- Car seats and carriers
- Furniture
- Teethers, soothers, dummies, or pacifiers that do not ease teething pain. There are regulated by the Consumer Product Safety Commission (CPSC).
- Baby and Maternity Class I Medical Devices
- Manual and electronic toothbrushes
- Dental care kits
- Baby sunglasses
- Baby and Maternity Class II Medical Devices
- Nasal aspirators
- Ear syringe sets
- Breast pumps and accessories
- Teethers, soothers, dummies, or pacifiers that ease teething pain. These are regulated by the US Food and Drug Administration (FDA)
- Baby and Maternity Sterilizers
- Baby bottle warmers and coolers
- Baby bottle sterilizers
- Baby clothing sterilizers
- Baby Skincare Products
- Baby sunscreen
- Baby oils, lotions, and creams
- Baby body washes, shampoos, and conditioners
- Baby wipes and wipe refills
- Electronic Children's Products
Exempt Products
Some items within the baby and maternity products category do not require certification. These products are not primarily designed or intended for children 12 years of age or younger.Requirements To Sell Baby and Maternity Products
You may be required to pass a category qualification process to sell Baby and Maternity Products on our platform. This process involves submitting documentation that shows you are qualified to sell in this category.Detailed requirements are outlined in collapsible sections below. Please expand each section to view the necessary information.
Baby and Maternity Products (Typical)
Manufacturers, Importers, And Repackers
If you are applying to sell Baby and Maternity Products you manufactured, imported, or repacked, you may be required to submit the following documents:- Children's Product Certificate (CPC)

- The manufacturer or importer’s name and address
- The date of issue (must be within the last 365 days)
- Product details that match the product category and listing photos
- A clear description of the tested product
- The name and address of the CPSC-accredited laboratory
- All relevant safety standards tested
- The certificate must be in English
- Test Report Issued by a CPSC-Accredited Laboratory

When submitting the test report, ensure it is legible, unaltered, and includes the following information:
- The manufacturer or importer’s name and address
- The name and address of the CPSC-accredited laboratory
- The date of issue (must be within the last 365 days)
- A clear description of the tested product
- Product details that match the product category and listing photos
- All relevant safety standards tested, with pass or fail results clearly shown
- The report must be in English
- Product Photos with Tracking Label
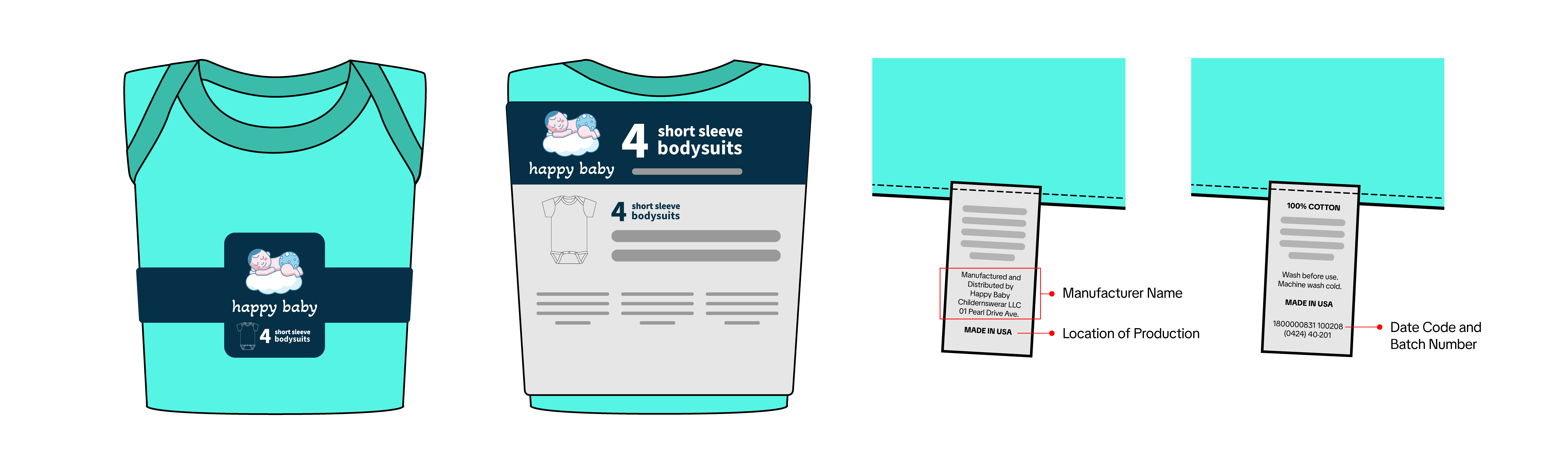
- Show all sides of the product
- Ensure all packaging information is visible, including product descriptions, warnings, and other relevant details
- Include a clear and legible image of the tracking label
- The manufacturer, importer, or private labeler’s name
- The location and date of production
- Detailed manufacturing information (e.g., batch number or run number) or other identifying characteristics
- Additional information identifying the product’s source
- The label must be in English
Resellers
If you are applying to sell Baby and Maternity Products as a reseller, you may be required to submit the following documents:- Children's Product Certificate (CPC)

- The manufacturer or importer’s name and address
- The date of issue (must be within the last 365 days)
- Product details that match the product category and listing photos
- A clear description of the tested product
- The name and address of the CPSC-accredited laboratory
- All relevant safety standards tested
- The certificate must be in English
- Product Photos with Tracking Label
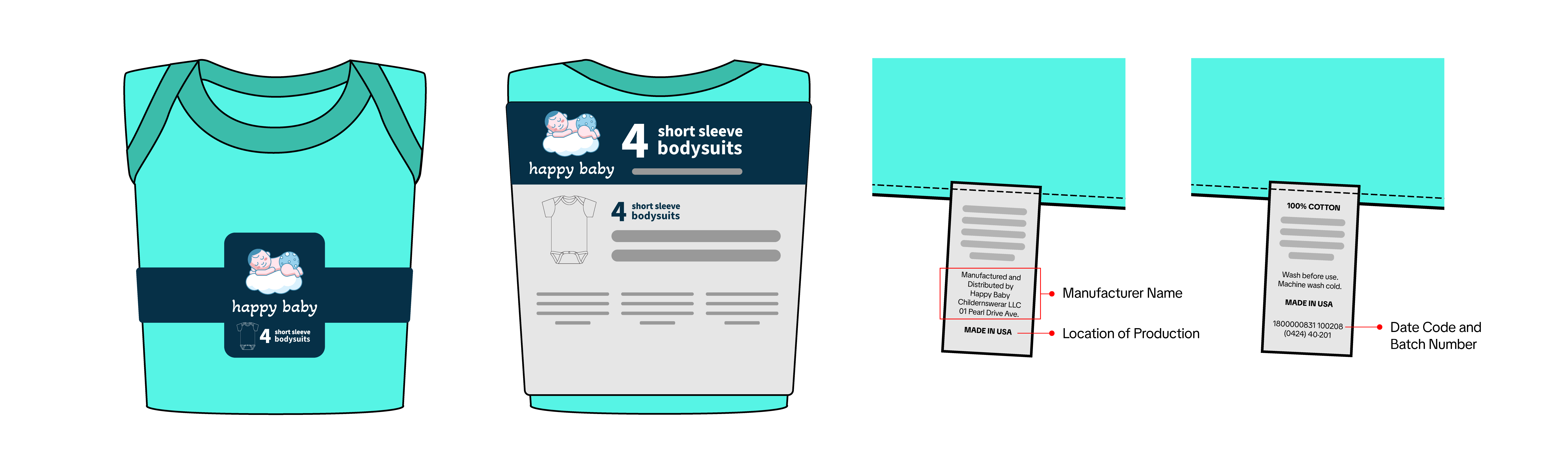
- Show all sides of the product
- Ensure all packaging information is visible, including product descriptions, warnings, and other relevant details
- Include a clear and legible image of the tracking label
- The manufacturer, importer, or private labeler’s name
- The location and date of production
- Detailed manufacturing information (e.g., batch number or run number) or other identifying characteristics
- Additional information identifying the product’s source
- The label must be in English
- Purchase Invoice
- The supplier’s name and address
- The date of issue (must be within the last 365 days)
- Product details and quantities that match the products being applied to sell
- The invoice must be in English
Baby & Maternity Medical Devices Class I
Manufacturers, Importers, And Repackers
If you are applying to sell Baby and Maternity Medical Devices Class I that you manufactured, imported, or repacked, you may be required to submit the following documents:- Proof of FDA Establishment Registration & Device Listing
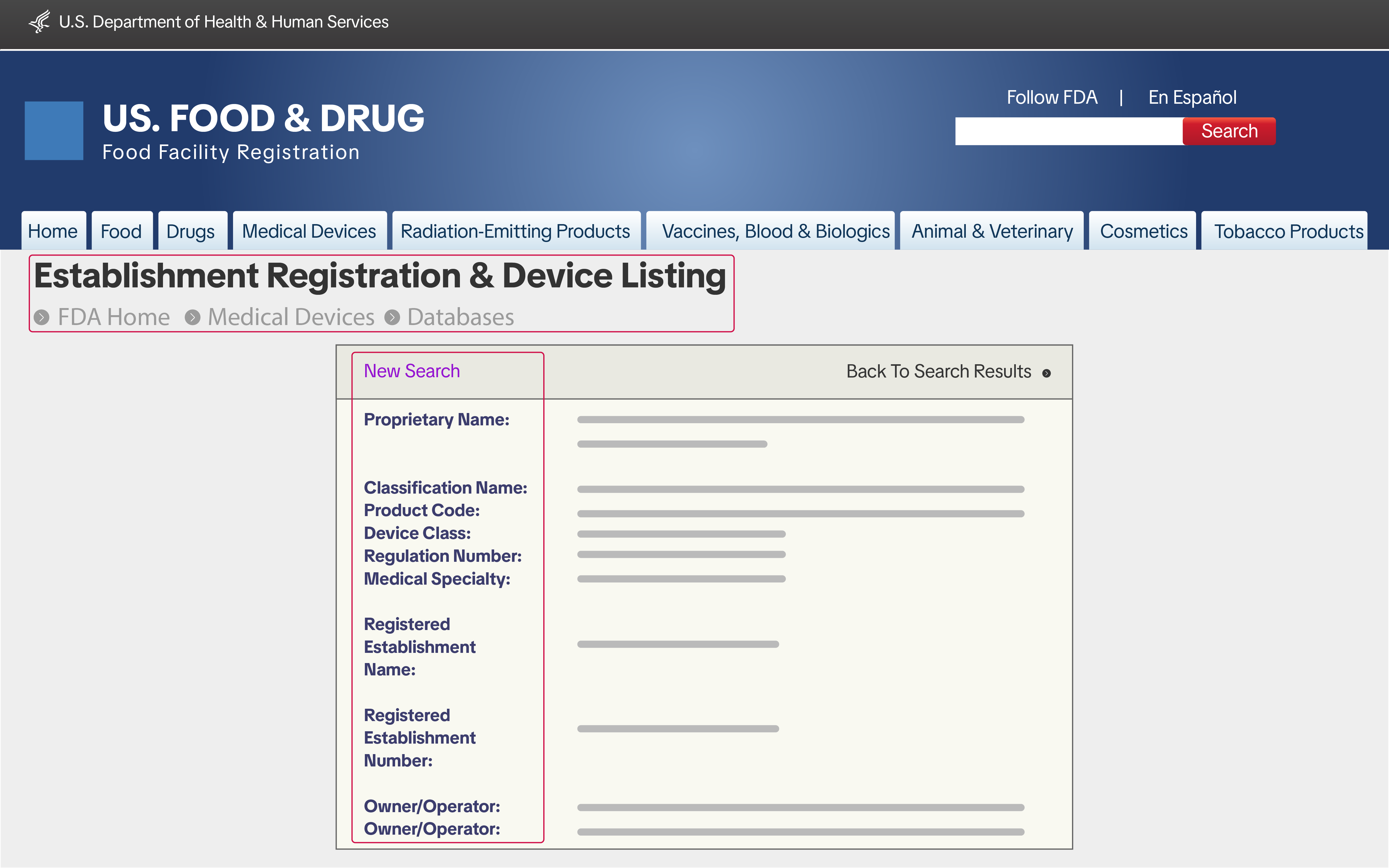
- A screenshot of the FDA Establishment Registration number (21 CFR Part 807) from the FDA Unified Registration and Listing System (FURLS)
- A screenshot of the FDA Device Listing (21 CFR Part 807) from FURLS
- The name and physical address of the registered establishment
- The operation type of the establishment
- Confirmation that the registration is valid at the time of submission
- Screenshots must clearly show that they were taken from the official FDA FURLS website
- All documents must be authentic and remain in their original (unmodified) format
- Product Label Images

The images must meet the following requirements:
- Show all sides of the product and packaging
- Be clear, legible, complete, unedited, and in color
- Have a minimum resolution of 600 × 600 pixels
- Avoid black-and-white, angled, blurry, cropped, or edited images
- Ensure all information on the product and packaging is visible and legible, including product description, warning logos, and any other required information
- Children's Product Certificate (CPC)

- The manufacturer or importer’s name and address
- The date of issue (must be within the last 365 days)
- Product details that match the product category and listing photos
- A clear description of the tested product
- The name and address of the CPSC-accredited laboratory
- All relevant safety standards tested
- The certificate must be in English
- Test Report Issued by a CPSC-Accredited Laboratory

When submitting the test report, ensure it is legible, unaltered, and includes the following information:
- The manufacturer or importer’s name and address
- The name and address of the CPSC-accredited laboratory
- The date of issue (must be within the last 365 days)
- A clear description of the tested product
- Product details that match the product category and listing photos
- All relevant safety standards tested, with pass or fail results clearly shown
- The report must be in English
- Electrical Safety Markings (if applicable)

- A valid electrical safety marking, either embossed on the product or applied as a sticker.
- The marking can also appear on the packaging.
- Stickers must be flat, with no creases or peeling edges.
- Double stickers are not allowed.
- For Bluetooth- or WiFi-enabled devices, include a photo of the FCC marking.
Resellers
If you are applying to sell Baby and Maternity Medical Devices as a reseller, you may be required to submit the following documents:- Purchase Invoice or Proof of Purchase

- Be dated within the last 180 days
- Show the same name and address as the selling account
- Include the full name and address of the manufacturer or distributor
- Contain products belonging to the applicable category
- Reflect a combined purchase of at least 400 units
- Be written in English or Chinese
- Pricing information may be omitted (optional)
- TikTok Shop reserves the right to verify the submitted documentation by contacting the product vendor listed on the invoice
- Retail order confirmations or invoices are not accepted
- Product Label Images

The images must meet the following requirements:
- Show all sides of the product and packaging
- Be clear, legible, complete, unedited, and in color
- Have a minimum resolution of 600 × 600 pixels
- Avoid black-and-white, angled, blurry, cropped, or edited images
- Ensure all information on the product and packaging is visible and legible, including product description, warning logos, and any other required information
- Children's Product Certificate (CPC)

- The manufacturer or importer’s name and address
- The date of issue (must be within the last 365 days)
- Product details that match the product category and listing photos
- A clear description of the tested product
- The name and address of the CPSC-accredited laboratory
- All relevant safety standards tested
- The certificate must be in English
- Electrical Safety Markings (if applicable)

- A valid electrical safety marking, either embossed on the product or applied as a sticker.
- The marking can also appear on the packaging.
- Stickers must be flat, with no creases or peeling edges.
- Double stickers are not allowed.
- For Bluetooth- or WiFi-enabled devices, include a photo of the FCC marking.
Baby & Maternity Sterilizers

Manufacturers, Importers, And Repackers
If you are applying to sell Baby and Maternity Sterilizers that you manufactured, imported, or repacked, you may be required to submit the following documents:- Product Photos
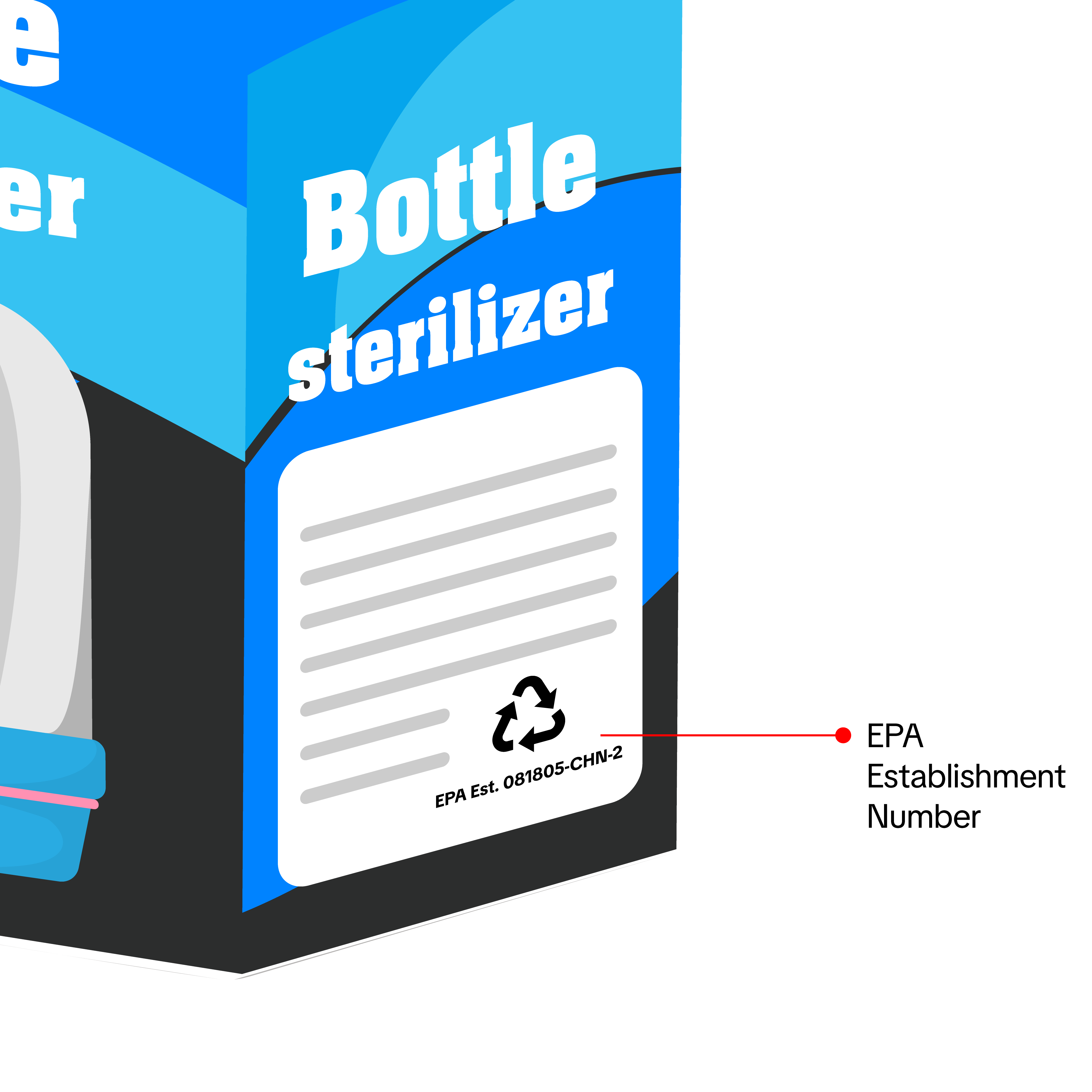
- Show all sides of the product.
- Sterilizers or products claiming sterilization must display an EPA Establishment Number on the label or packaging.
- Products without sterilization claims (e.g., bottle warmers) do not need an EPA Establishment Number.
- Electrical Safety Markings

- A valid electrical safety marking, either embossed on the product or applied as a sticker.
- The marking can also appear on the packaging.
- Stickers must be flat, with no creases or peeling edges.
- Double stickers are not allowed.
- For Bluetooth- or WiFi-enabled devices, include a photo of the FCC marking.
Resellers
If you are applying to sell Baby and Maternity Sterilizers as a reseller, you may be required to submit the following documents:- Product Photos

- Show all sides of the product.
- Sterilizers or products claiming sterilization must display an EPA Establishment Number on the label or packaging.
- Products without sterilization claims (e.g., bottle warmers) do not need an EPA Establishment Number.
- Electrical Safety Markings

- A valid electrical safety marking, either embossed on the product or applied as a sticker.
- The marking can also appear on the packaging.
- Stickers must be flat, with no creases or peeling edges.
- Double stickers are not allowed.
- For Bluetooth- or WiFi-enabled devices, include a photo of the FCC marking.
- Purchase Invoice

- The supplier’s name and address
- The date of issue (must be within the last 365 days)
- Product details and quantities that match the products being applied to sell
- The invoice must be in English
Baby Skincare Products
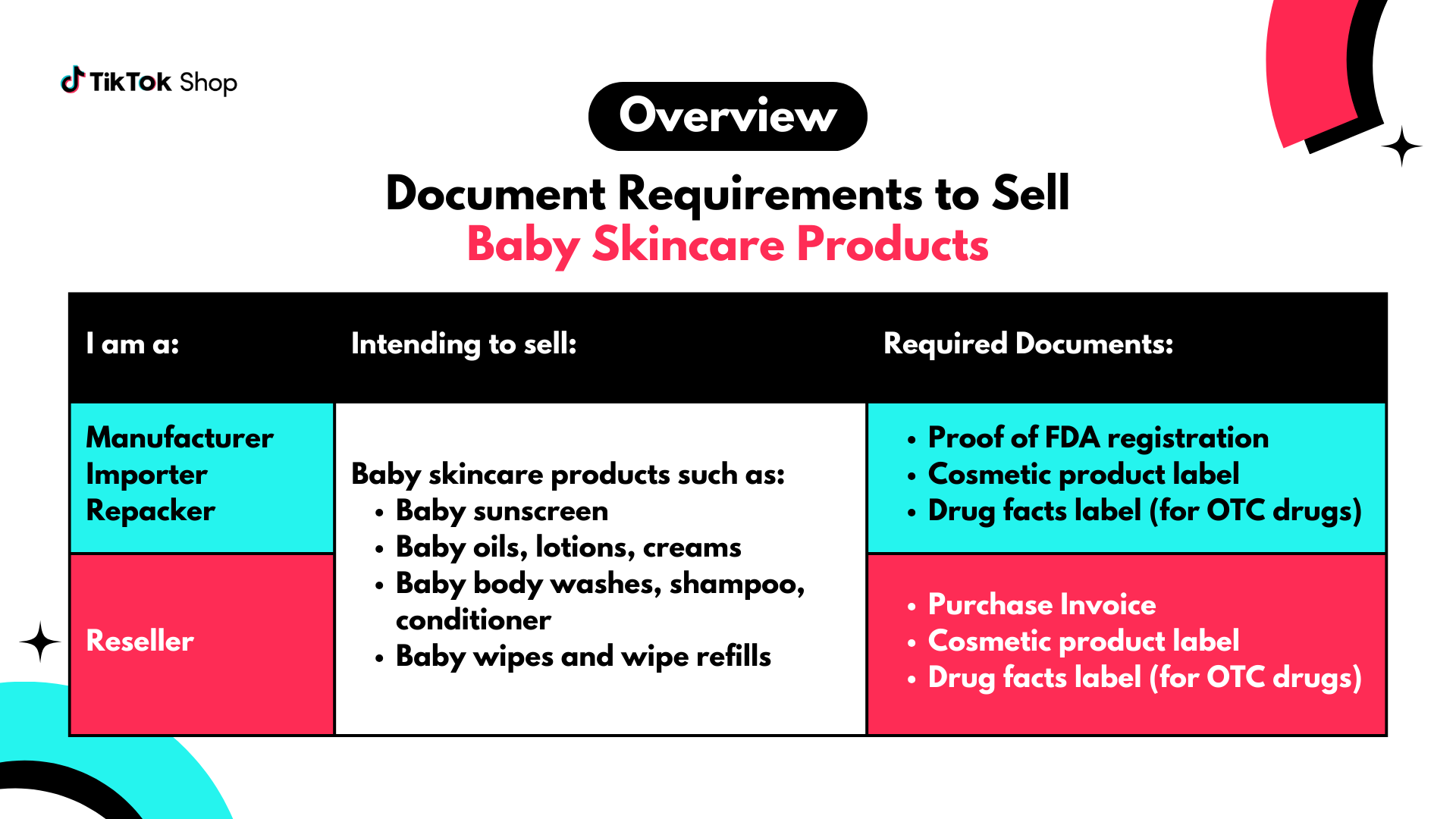
Manufacturers, Importers, And Repackers
If you are applying to sell Baby Skincare Products that you manufactured, imported, or repacked, you may be required to submit the following documents:- Proof of FDA Registration
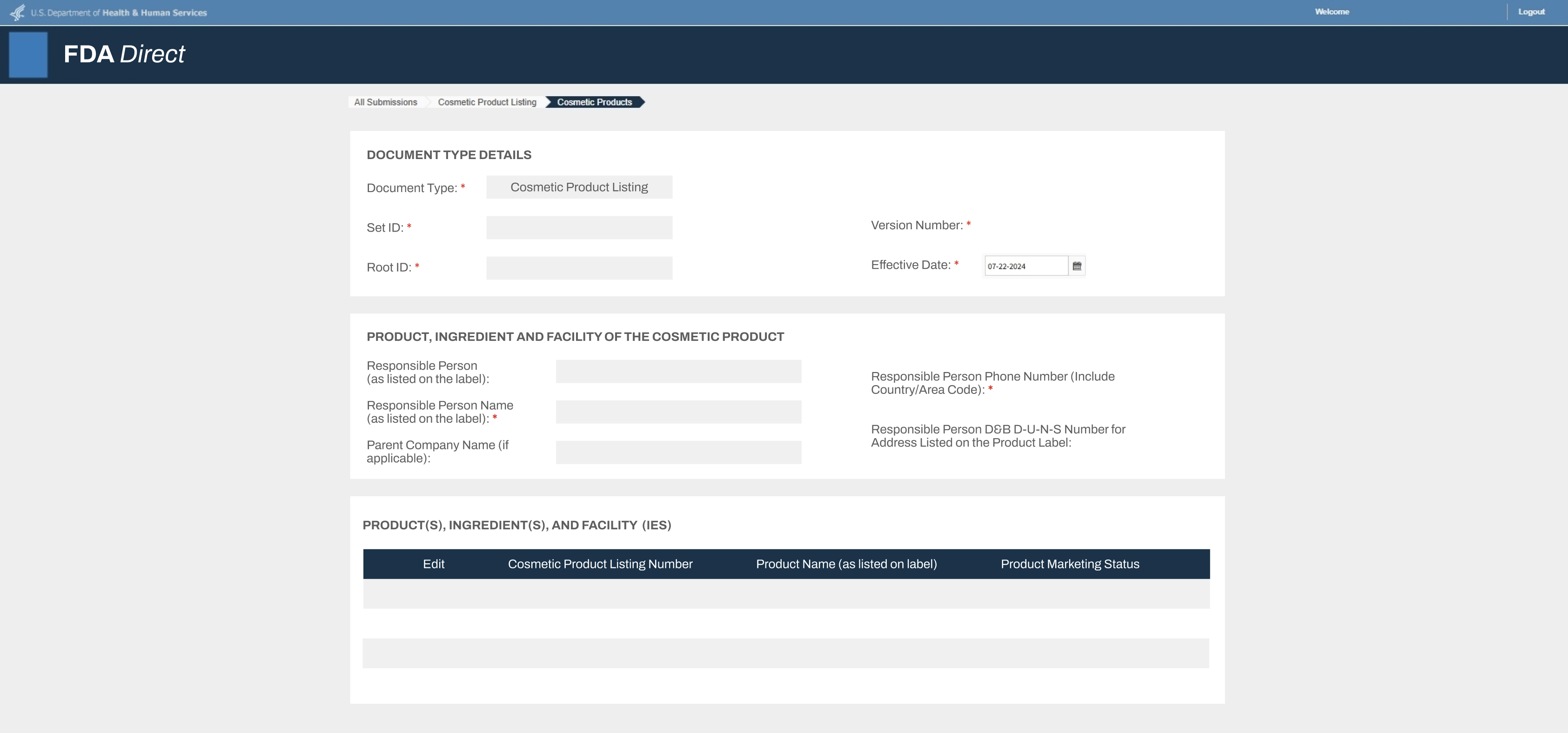
- Name of establishment. The name must match with the manufacturer, importer, or repacker as indicated on the product label
- Physical address of establishment
- Operation type of the registered establishment
- Registration number
- The FDA registration must be active at the time of submission
- FDA registration verification letter issued by a third-party
- If the FDA registration verification letter is issued by a third party, the third party name and contact information must be clearly identified on the verification letter.
- Screenshot of FDA registration portal
- If a screenshot of FDA registration is submitted, the image must be able to indicate that the screenshot is from the FDA website.
- If the seller qualifies for a MoCRA registration exemption (e.g., small businesses), they must provide self-attested documentation confirming their exemption status.
- This document should clearly state that the seller meets the criteria for exemption and is self-attesting to that eligibility.
- For more information, refer to the MoCRA Exemption Guidance.
- Cosmetic Product Label

- Principal display panel
- Product name
- Net contents
- Information panel
- Ingredient list
- Manufacturer, importer, or distributor's name and address
- Warning statements
- Drug Facts Label (if applicable)

- Product name
- Net contents
- Drug facts panel
- Active ingredients
- Inactive ingredients
- Direction or use instruction
- Warning statements
- Expiration date
- The expiration date must be presented with month, date, and year OR month and year. The month can be represented as numbers, a full word, or an abbreviation.
- Other information
Resellers
If you are applying to sell Baby Skincare Products as a reseller, you may be required to submit the following documents:- Purchase Invoice

- The supplier’s name and address
- The date of issue (must be within the last 365 days)
- Product details and quantities that match the products being applied to sell
- The invoice must be in English
- Cosmetic Product Label

- Principal display panel
- Product name
- Net contents
- Information panel
- Ingredient list
- Manufacturer, importer, or distributor's name and address
- Warning statements
- Drug Facts Label (if applicable)

- Product name
- Net contents
- Drug facts panel
- Active ingredients
- Inactive ingredients
- Direction or use instruction
- Warning statements
- Expiration date
- The expiration date must be presented with month, date, and year OR month and year. The month can be represented as numbers, a full word, or an abbreviation.
- Other information
Children's Electronic Products
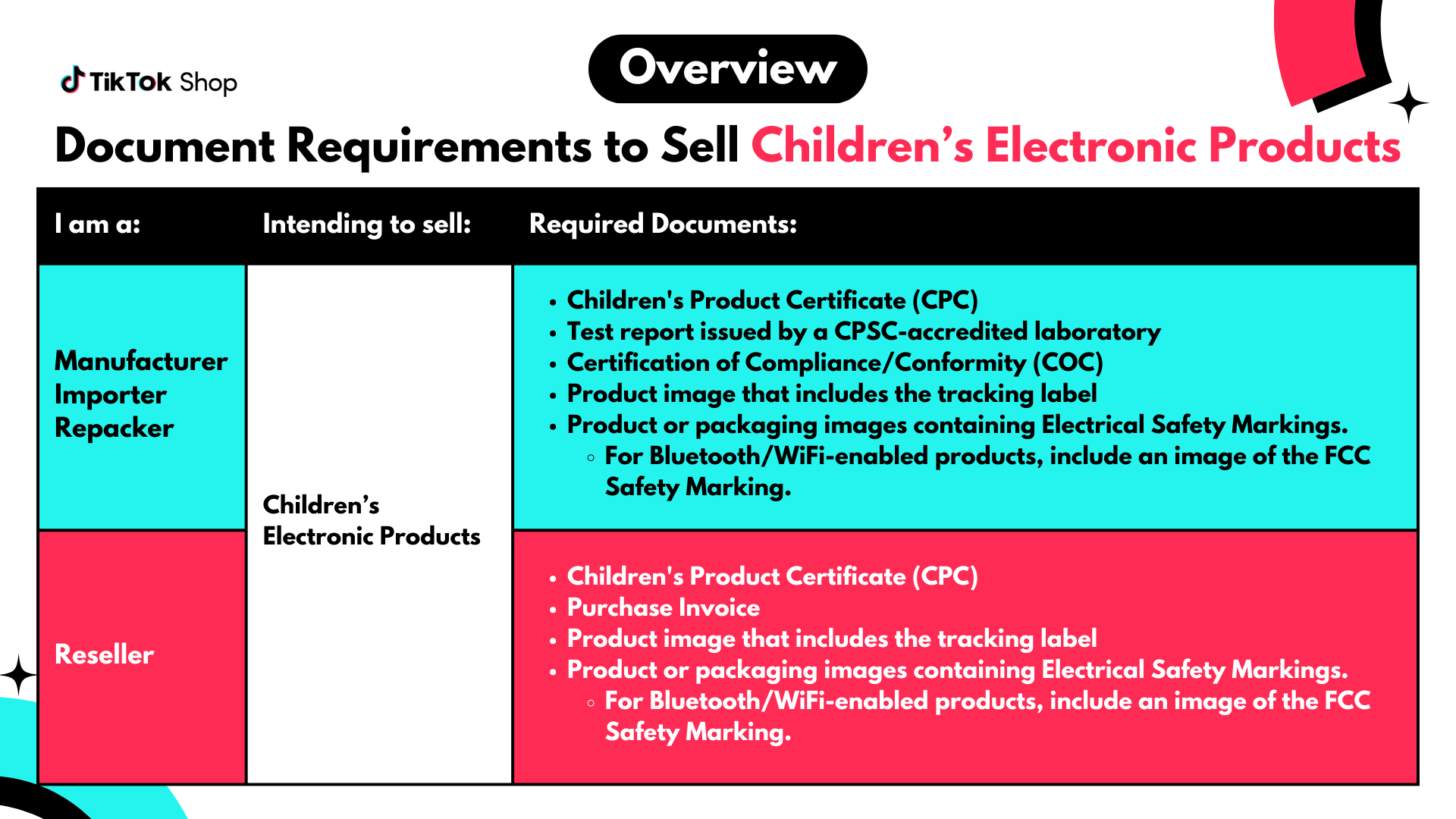
Manufacturers, Importers, And Repackers
If you are applying to sell Children's Electronic Products that you manufactured, imported, or repacked, you may be required to submit the following documents:- Children's Product Certificate (CPC)

- The manufacturer or importer’s name and address
- The date of issue (must be within the last 365 days)
- Product details that match the product category and listing photos
- A clear description of the tested product
- The name and address of the CPSC-accredited laboratory
- All relevant safety standards tested
- The certificate must be in English
- Test Report Issued by a CPSC-Accredited Laboratory

When submitting the test report, ensure it is legible, unaltered, and includes the following information:
- The manufacturer or importer’s name and address
- The name and address of the CPSC-accredited laboratory
- The date of issue (must be within the last 365 days)
- A clear description of the tested product
- Product details that match the product category and listing photos
- All relevant safety standards tested, with pass or fail results clearly shown
- The report must be in English
- Certification of Compliance or Conformity (COC)

- Issued within the past 2 years
- Matches the product category you intend to sell
- Includes a clear description and details of the product tested
- Lists all safety standards tested
- Includes the name and address of the accredited laboratory
- The product described must match the product photos provided
- Product Photos with Tracking Label
- Show all sides of the product
- Ensure all packaging information is visible, including product descriptions, warnings, and other relevant details
- Include a clear and legible image of the tracking label
- The manufacturer, importer, or private labeler’s name
- The location and date of production
- Detailed manufacturing information (e.g., batch number or run number) or other identifying characteristics
- Additional information identifying the product’s source
- The label must be in English
- Electrical Safety Markings

- A valid electrical safety marking, either embossed on the product or applied as a sticker.
- The marking can also appear on the packaging.
- Stickers must be flat, with no creases or peeling edges.
- Double stickers are not allowed.
- For Bluetooth- or WiFi-enabled devices, include a photo of the FCC marking.
Resellers
If you are applying to sell Children's Electronic Products as a reseller, you may be required to submit the following documents:- Children's Product Certificate (CPC)

- The manufacturer or importer’s name and address
- The date of issue (must be within the last 365 days)
- Product details that match the product category and listing photos
- A clear description of the tested product
- The name and address of the CPSC-accredited laboratory
- All relevant safety standards tested
- The certificate must be in English
- Purchase Invoice

- The supplier’s name and address
- The date of issue (must be within the last 365 days)
- Product details and quantities that match the products being applied to sell
- The invoice must be in English
- Product Photos with Tracking Label
- Show all sides of the product
- Ensure all packaging information is visible, including product descriptions, warnings, and other relevant details
- Include a clear and legible image of the tracking label
- The manufacturer, importer, or private labeler’s name
- The location and date of production
- Detailed manufacturing information (e.g., batch number or run number) or other identifying characteristics
- Additional information identifying the product’s source
- The label must be in English
- Electrical Safety Markings

- A valid electrical safety marking, either embossed on the product or applied as a sticker.
- The marking can also appear on the packaging.
- Stickers must be flat, with no creases or peeling edges.
- Double stickers are not allowed.
- For Bluetooth- or WiFi-enabled devices, include a photo of the FCC marking.
Choking Hazard Warning Label
⚠️
Children’s products that meet the U.S. Consumer Product Safety Commission (CPSC) criteria for choking hazards must include a clear choking hazard warning label. TikTok Shop requires all sellers to:
- Ensure the choking hazard warning is clearly displayed in the product listing.
- Include a compliant warning label on product packaging.
- Toys with small, detachable parts
- Puzzles with pieces smaller than 1.25 inches
- Jewelry or accessories intended for young children
- Art supplies such as beads or buttons
Even non-toy products may require this warning if they contain small parts that could detach and be swallowed, such as certain jewelry.
For more information, refer to the Choking Hazard Warning Label Policy.Enforcement Actions and Appeals
Claiming electrical safety without approved testing or certification violates this policy. Tests and certifications must come from recognized electrical safety or FCC laboratories. Products are also in violation if they have inaccurate or fraudulent labeling.
We regularly review your shop’s compliance with this policy. If any violations are identified, TikTok Shop may take enforcement action at our sole discretion. This may include, but is not limited to:- Rejecting your category qualification application
- Deducting points from your account health
- Removing product listings
How to Submit Your Documentation
Submit your documentation via the Qualification Center in Seller Center. Click here or follow these steps:- Log into your Seller Center account.
- Click your shop icon in the top right corner.
- Go to My Account > Account Settings.
- Select Qualification Center.
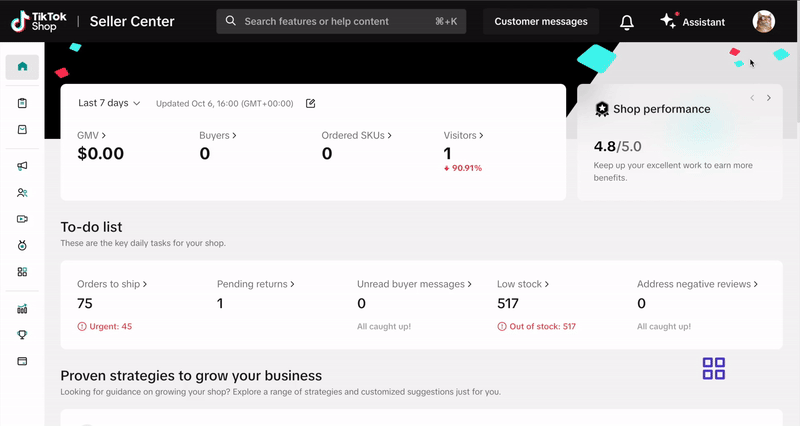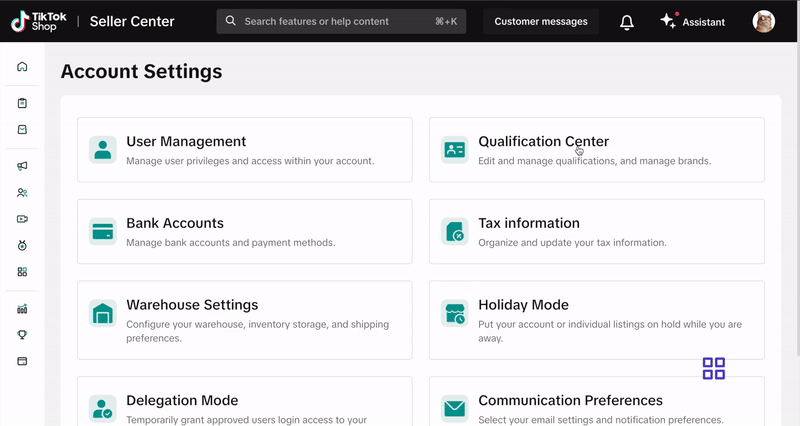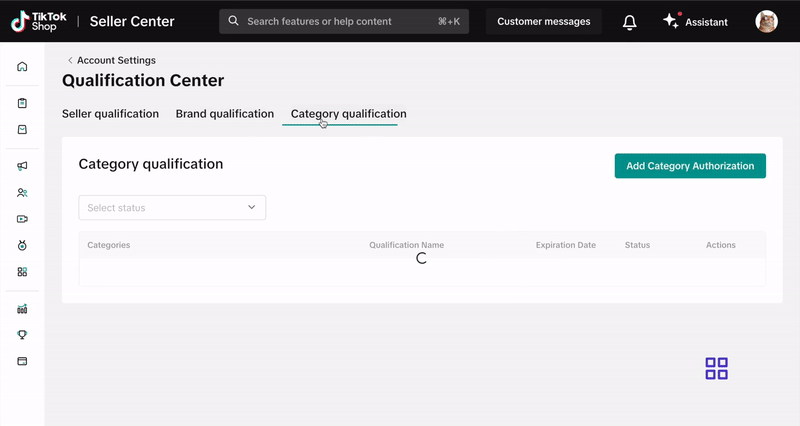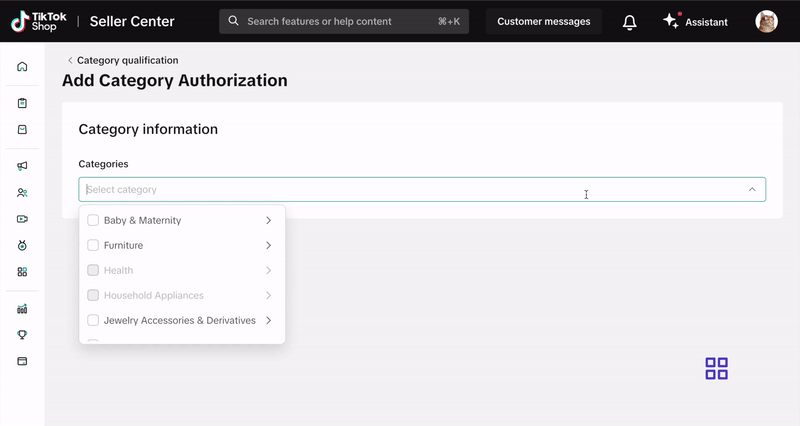
- Click on Category Qualification.
- Click Add Category Authorization and follow the prompts to submit your application.
How To Address A Category Qualification Rejection
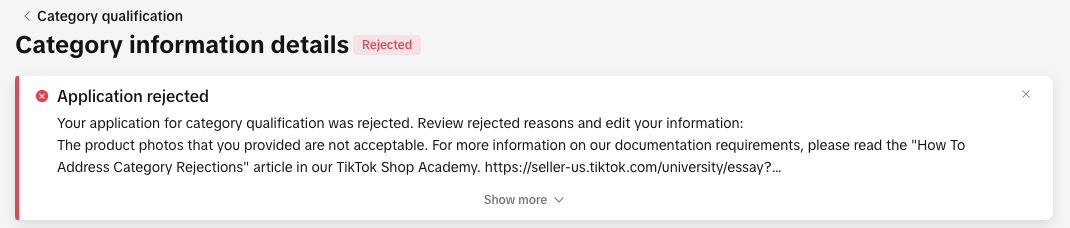
You can view your rejection message by:
- Clicking the bell icon at the top of your Seller Center homepage to go to your inbox
- Opening the rejected application in the Qualification Center.
- Go to the Qualification Center, then click Category Qualification.
- Click the rejected category to view the rejection reason.
- Review the related category policy to confirm what’s needed
- Update or replace your documentation to meet the category’s requirements.
- Click Resubmit to submit your revised application.