Dietary Supplements Policy
01/20/2026

This policy outlines the requirements for selling Dietary Supplements on TikTok Shop. Eligibility to sell dietary supplements is limited to invite-only sellers. Contact your Account Manager (AM) to learn more.Key Points:
- To sell Dietary Supplements, you must be invited to offer products in the category. Contact your Account Manager (AM) to learn more.
- For help with the review process and rejection handling, see Your Guide to Category Qualification.
Dietary Supplements
A dietary supplement is a product meant for oral ingestion. It contains an ingredient intended to support your diet. This ingredient can include vitamins, minerals, herbs, botanicals, and amino acids.Permitted Dietary Supplements
The following dietary supplements are allowed to be sold on our platform:- Beauty supplements
- Fitness supplements
- Vitamins, mineral and wellness supplements
- Maternity supplements
- Herbal supplements
- Sexual wellness supplements, such as testosterone boosters and sexual performance enhancer supplements. See Promoting Sexual Wellness Products for more information.
Prohibited Dietary Supplements
The following dietary supplements are prohibited on our platform:- Weight management supplements, such as appetite suppressants, carbohydrate control supplements, diet shakes, fat burners, meal replacement bars, meal replacement drinks, and weight loss bars.
- Mental wellness supplements
- Sexual performance enhancers
Requirements To Sell Dietary Supplements
Select sellers will be invited into this category by their Account Manager (AM). Invited sellers will need to submit documents proving they are qualified to sell these products.Detailed requirements are listed in the sections below. Open each section to view what is needed.
Manufacturers, Importers, And Repackers
If you are applying to sell dietary supplements you manufactured, imported, or repacked, you must submit the following documents:
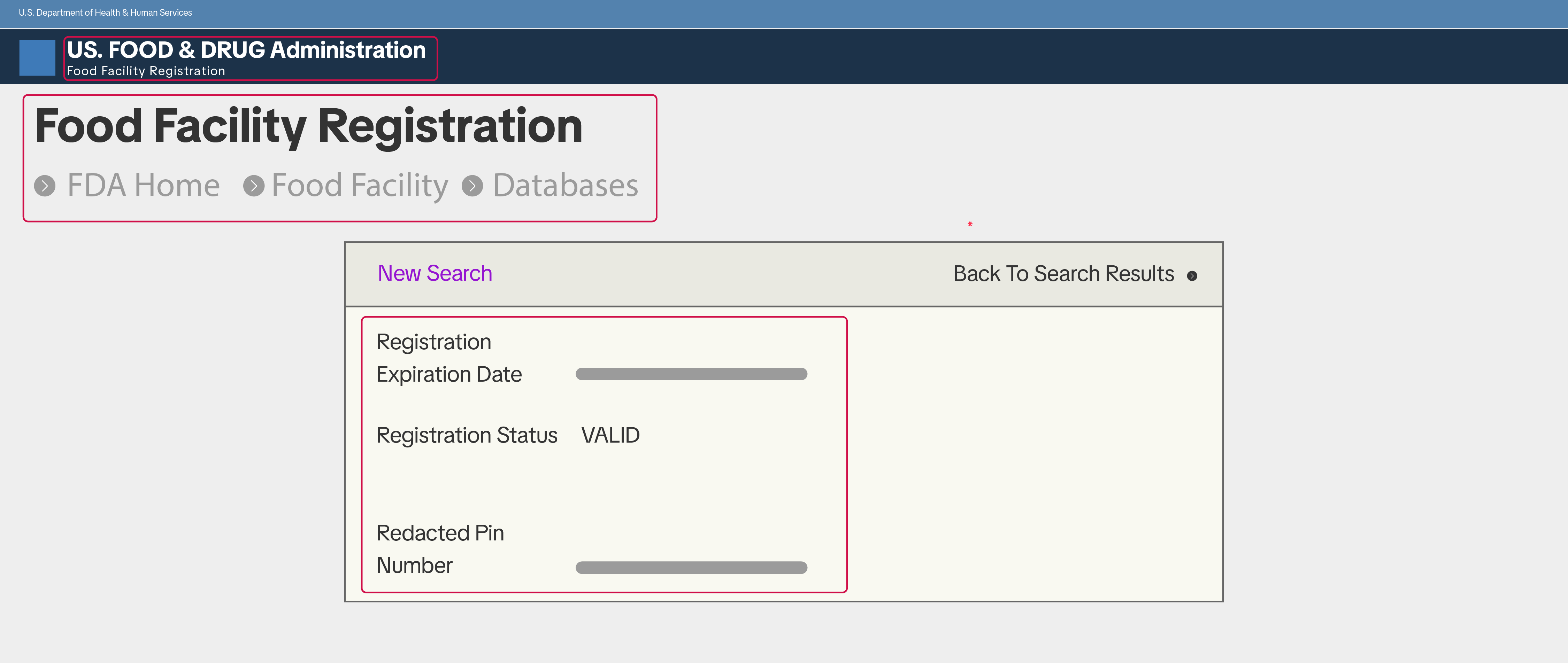 Manufacturers, importers, and repackers may be required to provide one of the following:
Manufacturers, importers, and repackers may be required to provide one of the following:
 cGMP refers to the current Good Manufacturing Practice (cGMP) regulations enforced by the FDA. Manufacturers must submit proof of cGMP certification that meets the following requirements:
cGMP refers to the current Good Manufacturing Practice (cGMP) regulations enforced by the FDA. Manufacturers must submit proof of cGMP certification that meets the following requirements:
 You must submit images of the front, back, and supplement facts label of a sample dietary supplement you intend to sell on our platform. All labels must be clearly visible on the product.
You must submit images of the front, back, and supplement facts label of a sample dietary supplement you intend to sell on our platform. All labels must be clearly visible on the product.
The front label must include:
- Proof Of FDA Food Facility Registration

- A verification letter from a third party confirming valid FDA food facility registration
- A screenshot from the FDA Direct website showing valid FDA food facility registration
- The name of the registered establishment (must match the manufacturer or importer listed on the product label)
- The physical address of the registered establishment
- The operation type of the establishment
- Confirmation that the registration is valid at the time of submission
- If submitting a screenshot, it must clearly show that it was taken from the official FDA website
- If submitting a third-party verification letter, the letter must clearly display the third party’s name and contact information
- Proof of cGMP Certification (Only for Manufacturers)

- The certification must be valid at the time of submission
- It must confirm that the establishment complies with 21 CFR Part 111
- Product Lab Test Report
- A valid test report from TikTok Shop Accredited Partner Laboratories (listed below); or
- A valid test report from an in-house ISO/IEC 17025–accredited laboratory, plus a valid ISO certificate; or
- A valid test report from an in-house laboratory, plus a valid cGMP certificate compliant with 21 CFR 111 or 21 CFR 117.
- Eurofins
- NSF (National Sanitation Foundation) International
- SGS (General Society of Surveillance)
- UL (Underwriters Laboratories) Solutions
- TÜV Sud
- Mérieux NutriSciences
- Intertek
- Certified Laboratories
- Issued within the last 180 days
- Cover products that match the purchase invoice, including brand and manufacturer details
- Display the same company name and address as the selling account
- If the seller is the manufacturer, the manufacturer listed on the report must match the selling account
- Microbiological testing
- Heavy metals testing
- Contaminant testing, such as pesticides or residual solvents (as labeled on the report)
- Content claims / potency / label claim verification
- The reported amount of each active ingredient per dosage unit must fall within the permitted tolerance range
- Images Of Sample Product Labels

The front label must include:
- Product name
- Net weight or quantity
- Type of supplement
- List of ingredients
- Serving size
- Name and physical address of the product’s manufacturer or distributor
Resellers
If you are applying to sell dietary supplements as a reseller, you must submit the following documents:
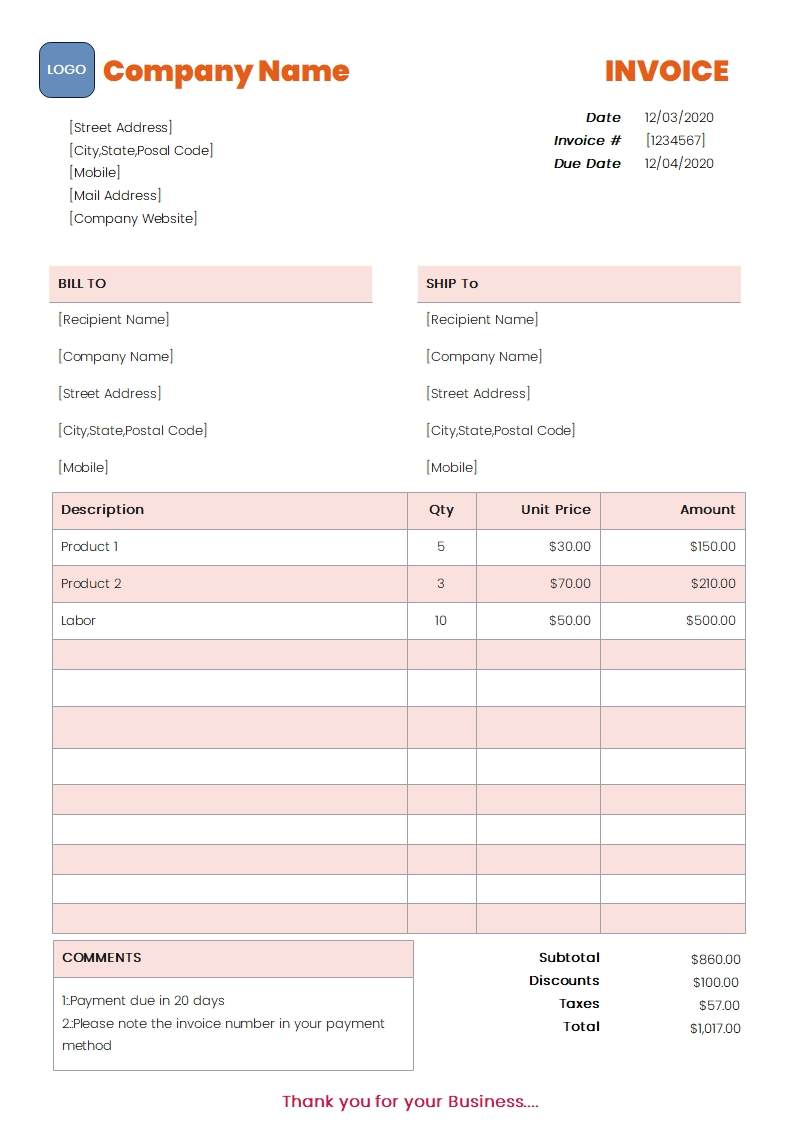 Resellers must submit a legible purchase invoice or proof of purchase issued by the product’s manufacturer or distributor. The invoice must meet all of the following requirements:
Resellers must submit a legible purchase invoice or proof of purchase issued by the product’s manufacturer or distributor. The invoice must meet all of the following requirements:
Accepted Test Report TypesThe test report must be issued by a TikTok Shop Accredited Partner Laboratory, including:
 You must submit images of the front, back, and supplement facts label of a sample dietary supplement you intend to sell on our platform. All labels must be clearly visible on the product.
You must submit images of the front, back, and supplement facts label of a sample dietary supplement you intend to sell on our platform. All labels must be clearly visible on the product.
The front label must include:
- Purchase Invoice or Proof of Purchase

- Be dated within the last 180 days
- Show the same name and address as the selling account
- Include the full name and address of the manufacturer or distributor
- Contain products belonging to the applicable category
- Reflect a combined purchase of at least 400 units
- Be written in English or Chinese
- Pricing information may be omitted (optional)
- TikTok Shop reserves the right to verify the submitted documentation by contacting the product vendor listed on the invoice
- Retail order confirmations or invoices are not accepted
- Product Lab Test Report
Accepted Test Report TypesThe test report must be issued by a TikTok Shop Accredited Partner Laboratory, including:
- Eurofins
- NSF (National Sanitation Foundation) International
- SGS (General Society of Surveillance)
- UL (Underwriters Laboratories) Solutions
- TÜV Sud
- Mérieux NutriSciences
- Intertek
- Certified Laboratories
- Issued within the last 180 days
- Cover products that match the purchase invoice, including brand and manufacturer details
- Display the same company name and address as the selling account
- If the seller is the manufacturer, the manufacturer listed on the report must match the selling account
- Microbiological testing
- Heavy metals testing
- Contaminant testing, such as pesticides or residual solvents (as labeled on the report)
- Content claims / potency / label claim verification
- The reported amount of each active ingredient per dosage unit must fall within the permitted tolerance range
- Images Of Sample Product Labels

The front label must include:
- Product name
- Net weight or quantity
- Type of supplement
- List of ingredients
- Serving size
- Name and physical address of the product’s manufacturer or distributor
Infant & Toddler Supplements and Other Nutritional Products
Infant and toddler supplements and other nutritional products are subject to additional safety and compliance requirements due to their intended use for young children.Only invite-only sellers may list products in this category.
Permitted Infant & Toddler SupplementsOnly the following products may be listed. Products outside this list are not allowed at this time.
Infant Products
- Vitamin D drops
- Iron drops
- Probiotic or prebiotic supplements
- Digestive nutritional supplements
- Iron supplements
- Basic multivitamins
- Vitamin D drops
- Basic multivitamins
- Electrolyte drinks
- Probiotic or prebiotic drinks
- Treats, cures, or prevents diseases
- Replaces medical treatment
- Provides unapproved medical benefits
- Examples include “treats colic” or “cures reflux”
Required Information on Your Product ListingYour product page must clearly display:
- Intended age range
- Dosage form (drops, liquid, chewable, etc.)
- Active ingredients and strength per serving
- Key safety warnings
- Whether the product is classified as a dietary supplement or an OTC drug
- Front label
- Supplement Facts or Drug Facts panel
- Directions for use
- Warnings
- A valid test report from TikTok Shop Accredited Partner Laboratories (listed below); or
- A valid test report from an in-house ISO/IEC 17025–accredited laboratory, plus a valid ISO certificate; or
- A valid test report from an in-house laboratory, plus a valid cGMP certificate compliant with 21 CFR 111 or 21 CFR 117.
- Eurofins
- NSF (National Sanitation Foundation) International
- SGS (General Society of Surveillance)
- UL (Underwriters Laboratories) Solutions
- TÜV Sud
- Mérieux NutriSciences
- Intertek
- Certified Laboratories
- Issued within the last 180 days
- Cover products that match the purchase invoice, including brand and manufacturer details
- Display the same company name and address as the selling account
- If the seller is the manufacturer, the manufacturer listed on the report must match the selling account
- Microbiological testing
- Heavy metals testing
- Contaminant testing, such as pesticides or residual solvents (as labeled on the report)
- Content claims / potency / label claim verification
- The reported amount of each active ingredient per dosage unit must fall within the permitted tolerance range.
- FDA food facility registration
- cGMP certification
- ISO or HACCP certification (if applicable)
- Is correctly classified as a dietary supplement
- Is manufactured according to FDA requirements
- Uses only permitted ingredients
- Is properly labeled
- Is not unsafe or misleading
- Complies with FDA and FTC rules for marketing and claims
Enforcement Actions and Appeals
We regularly review your shop’s compliance with this policy. If any violations are identified, TikTok Shop may take enforcement action at our sole discretion. This may include, but is not limited to:- Removing your ability to sell in this category
- Deducting points from your account health
- Removing product listings