Pet Supplies Policy
12/21/2025

This policy outlines the requirements for selling Pet Supplies on TikTok Shop.Key Points:
- To sell Pet Supplies, you may need to submit documentation through the Qualification Center. Requirements vary depending on your role as a seller.
- For help with the review process and rejection handling, see Your Guide to Category Qualification.
Pet Supplies
The following Pet Supplies are allowed to be sold on our platform through category qualification.- Pet Food
- Pet Supplements
Electrical Pet Supplies
Please refer to our Electronic Products Policy for requirements to sell electrical pet supplies, such as:- Electric Clippers
- Habitat Lighting
- Hair Dryers
- Terrarium Heat Lamps
Prohibited Products
The following pet supplies are prohibited from sale on TikTok Shop:- Pet health products, which include, but are not limited to:
- Bird healthcare
- Flea and tick treatments
- Cat and dog medications
- Farm animal health supplies
- Fish and aquatic supplies
- Reptile and amphibian supplies
- Small animal supplies
- Grooming products, which include, but are not limited to:
- Colognes
- Finishing sprays
- Dander remover sprays
- Deodorizers
- Grooming scissors
- Styptic gels and powders
Requirements To Sell Pet Supplies
You may be required to pass category qualification to sell pet supplies. This process requires submitting documents proving you are qualified to sell these products.Detailed requirements are listed in the sections below. Open each section to view what is needed.
Pet Food
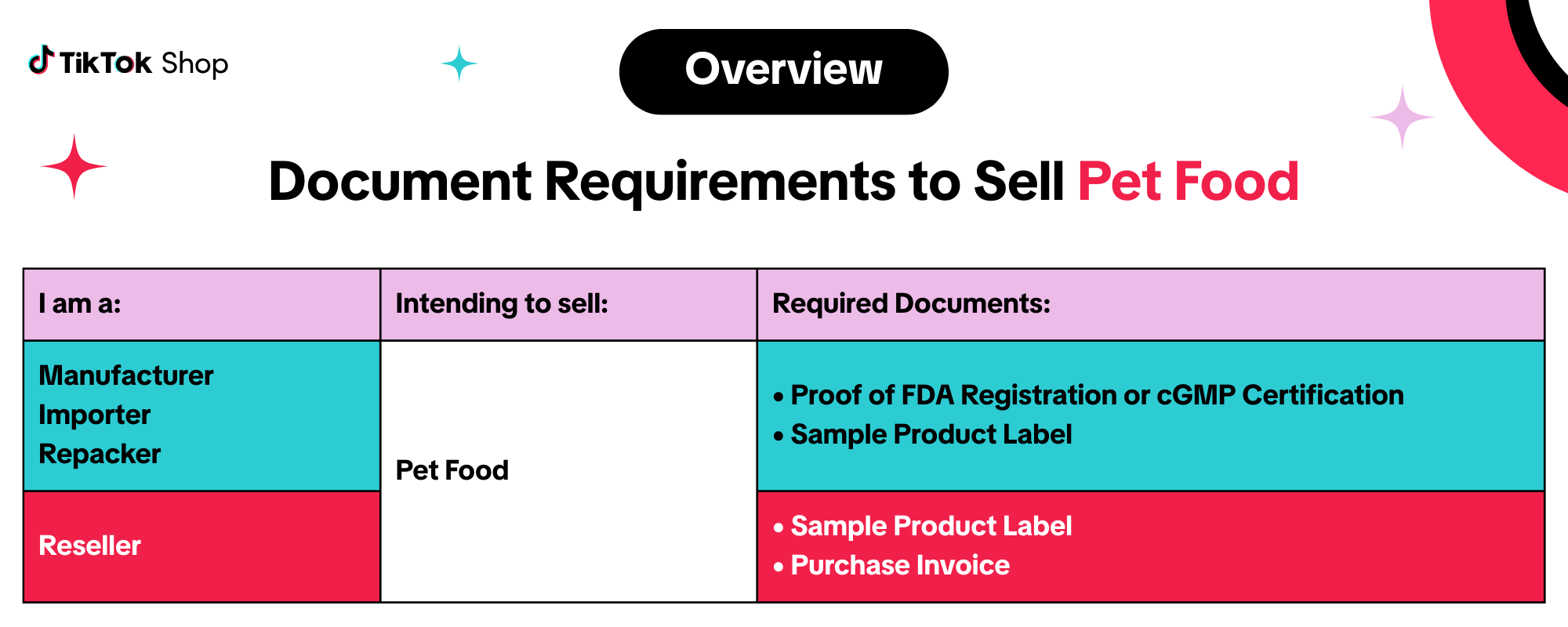
Manufacturers, Importers, And Repackers
If you are applying to sell pet food you manufactured, imported, or repacked, you may be required to submit the following documents:- Proof of FDA Registration or cGMP Certification
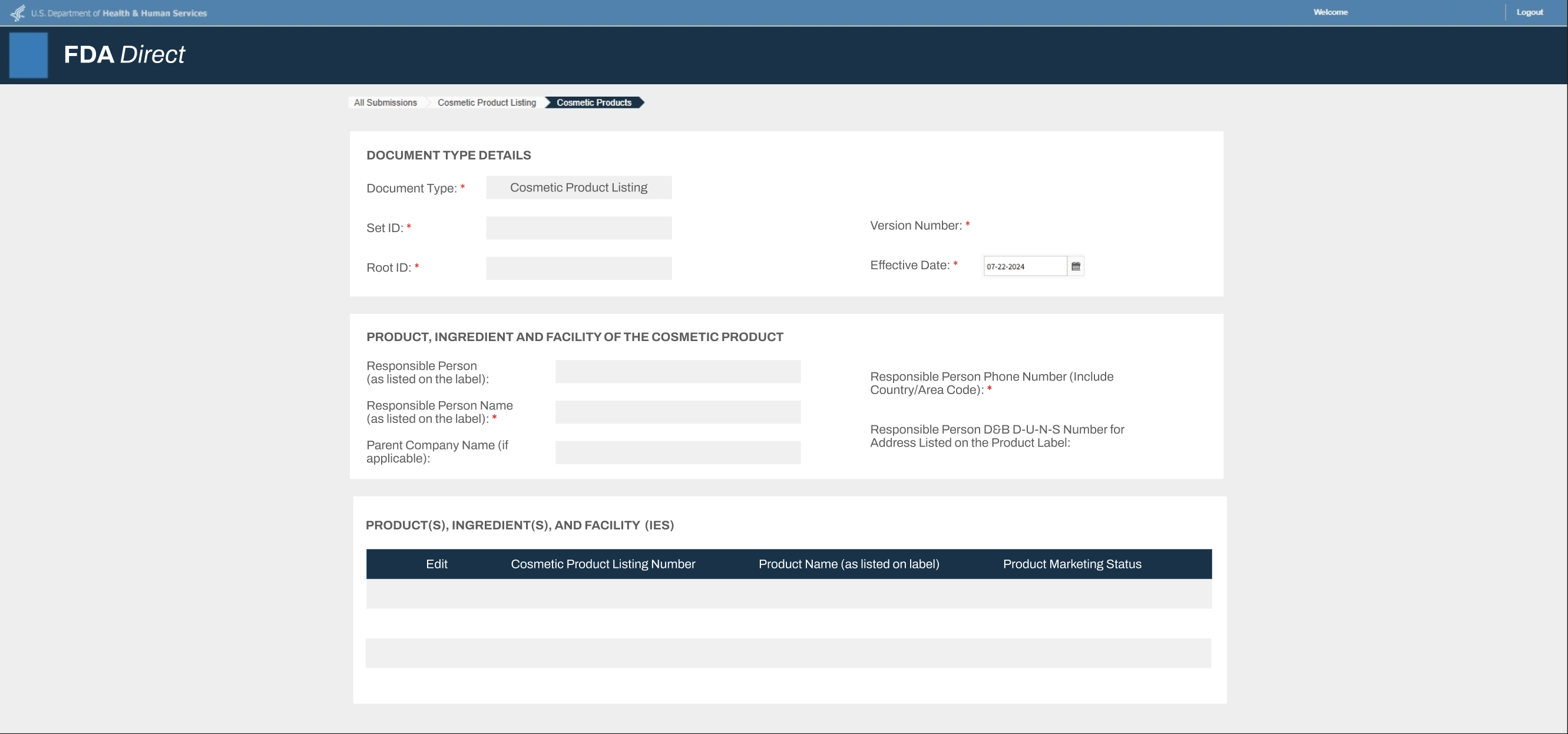
FDA RegistrationYou may be required to submit a third-party issued FDA registration verification letter or a screenshot of the FDA registration portal. The document must include:
- Name of establishment. The name must match with the manufacturer or importer indicated on the product label.
- Physical address of establishment
- Operation type of the registered establishment
- The FDA registration must be active at the time of submission.
- If a screenshot of the FDA registration is submitted, the image must be able to indicate the screenshot is from the FDA website.
- If the FDA registration verification letter is used by a third-party, the third-party name and contact information must be clearly identified on the verification letter.
The cGMP certificate must:
- Be valid and not expired at the time of submission
- Clearly state that the certified facility complies with 21 CFR Part 507
- Sample Product Label

- Product name and information
- Net quantity
- Manufacturer/distributor's name and address
- List of ingredients arranged by weight, from most to least
Resellers
If you are applying to sell pet food as a reseller, you may be required to submit the following documents:- Purchase Invoice

- Include the supplier’s company name and address
- Clearly describe the pet supplement being listed on TikTok Shop
- Be issued within the last 365 days
- Be written in a language supported by TikTok Shop
- Sample Product Label

- Product name and information
- Net quantity
- Manufacturer/distributor's name and address
- List of ingredients arranged by weight, from most to least
Pet Supplements
This section outlines the requirements for selling pet supplements, which includes the following:
- Digestive Remedies (Dogs & Cats)
- Amino Acids (Dogs & Cats)
- Antioxidants (Dogs & Cats)
- Fish Oil Supplements (Dogs & Cats)
- Herbal Supplements (Dogs & Cats)
- Multivitamins (Dogs & Cats)
- Probiotics (Dogs & Cats)
Manufacturers, Importers, And Repackers
If you are applying to sell pet supplements you manufactured, imported, or repacked, you may be required to submit the following documents.- Proof of FDA Registration
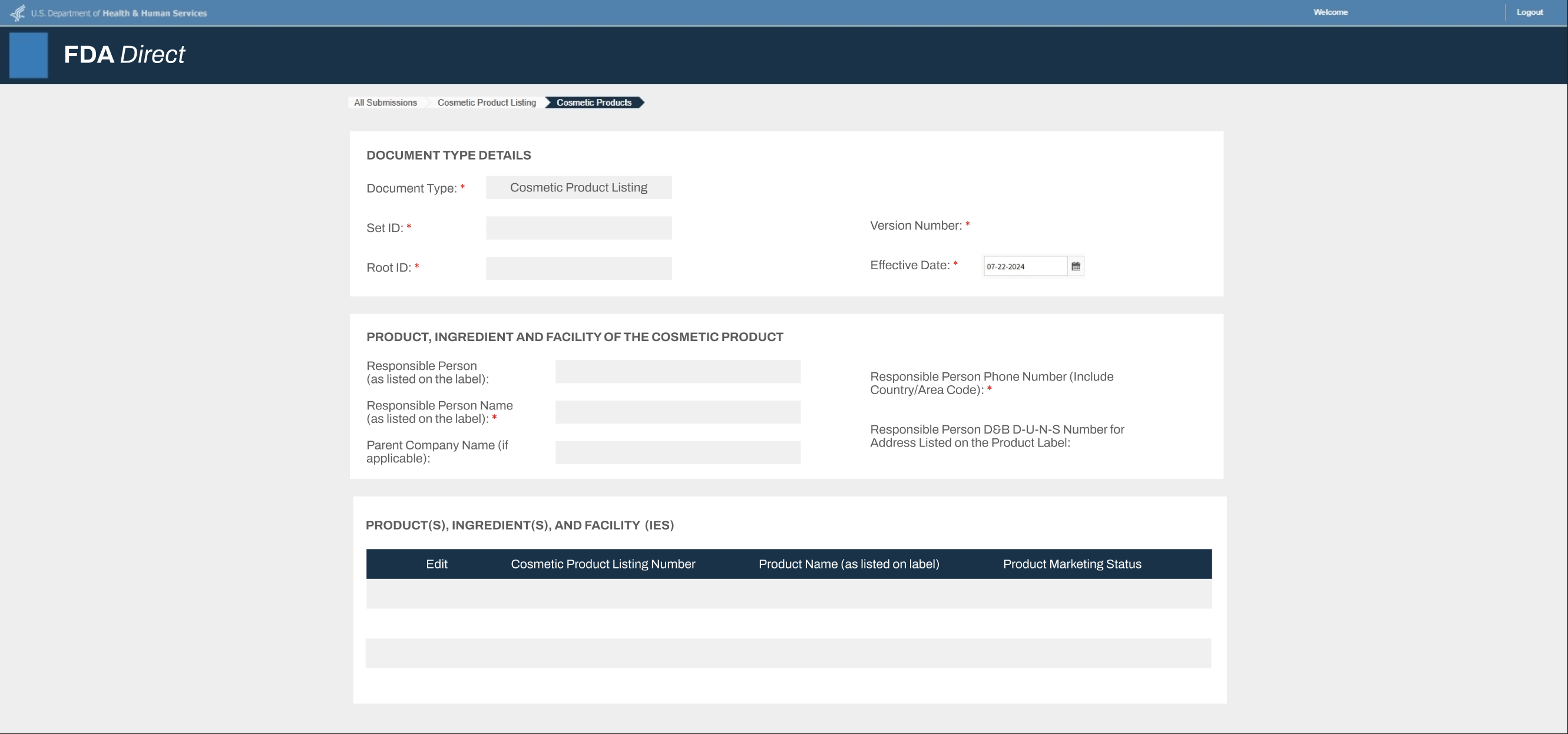
- Name of establishment. The name must match with the manufacturer or importer indicated on the product label.
- Physical address of establishment
- Operation type of the registered establishment
- The FDA registration must be active at the time of submission.
- If a screenshot of the FDA registration is submitted, the image must be able to indicate the screenshot is from the FDA website.
- If the FDA registration verification letter is used by a third-party, the third-party name and contact information must be clearly identified on the verification letter.
- cGMP Certification
The cGMP certificate must:
- Be valid and not expired at the time of submission
- Clearly state that the certified facility complies with 21 CFR Part 507
- Sample Product Label

The front label must include:
- Product name
- Name of the animal species the product is intended for
- Net weight/quantity
- Specification of type of supplement included
- Manufacturer or distributor's name and address
- Ingredients in the product
- Serving size
Resellers
If you are applying to sell pet supplements as a reseller, you may be required to submit the following documents:- Purchase Invoice
- Include the supplier’s company name and address
- Clearly describe the pet supplement being listed on TikTok Shop
- Be issued within the last 365 days
- Be written in a language supported by TikTok Shop
- Sample Product Label

The front label must include:
- Product name
- Name of the animal species the product is intended for
- Net weight/quantity
- Specification of type of supplement included
- Manufacturer or distributor's name and address
- Ingredients in the product
- Serving size
Enforcement Actions and Appeals
We regularly review your shop’s compliance with this policy. If any violations are identified, TikTok Shop may take enforcement action at our sole discretion. This may include, but is not limited to:- Rejecting your category qualification application
- Deducting points from your account health
- Removing product listings
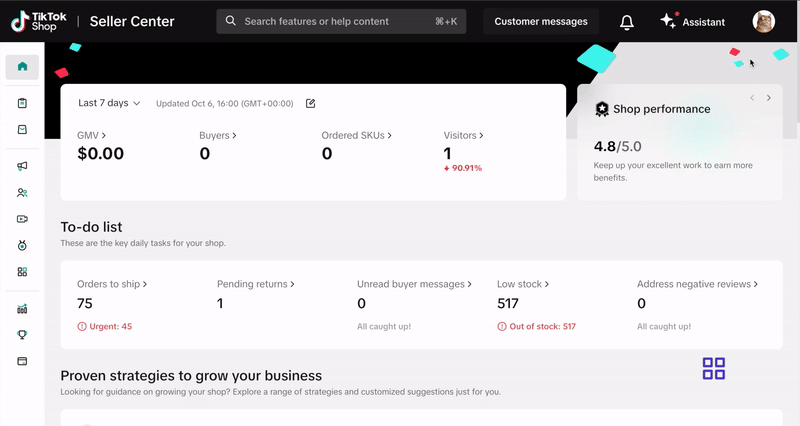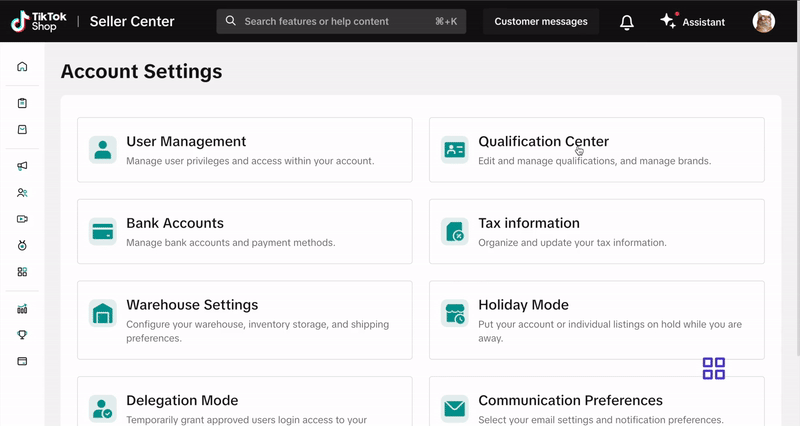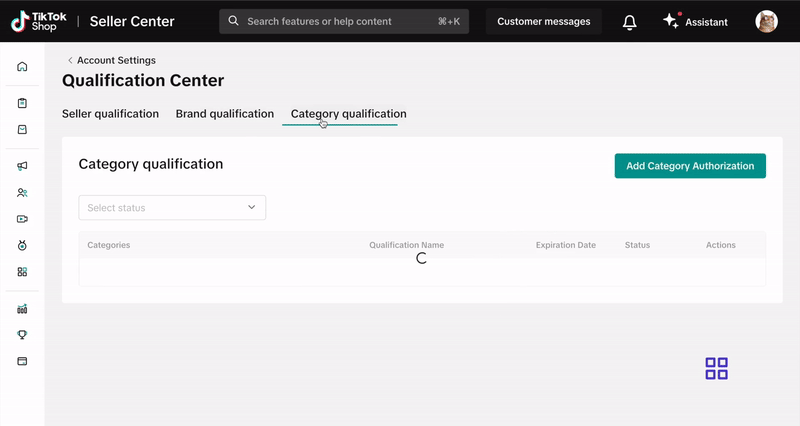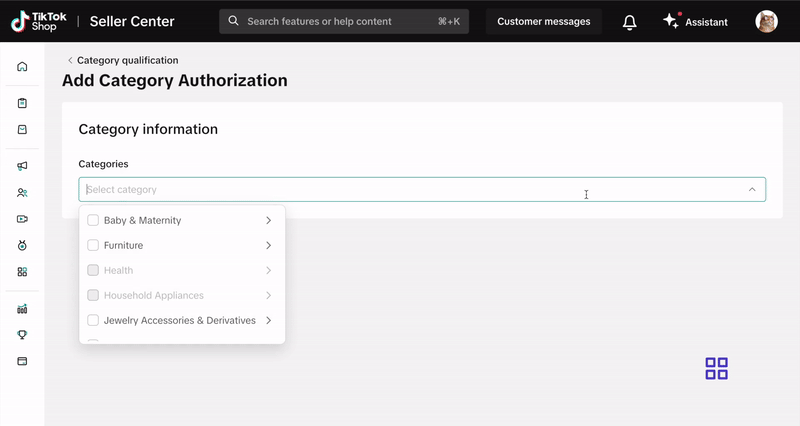
How to Submit Your Documentation
Submit your documentation via the Qualification Center in Seller Center. Click here or follow these steps:- Log into your Seller Center account.
- Click your shop icon in the top right corner.
- Go to My Account > Account Settings.
- Select Qualification Center.
- Click on Category Qualification.
- Click Add Category Authorization and follow the prompts to submit your application.
How To Address A Category Qualification Rejection
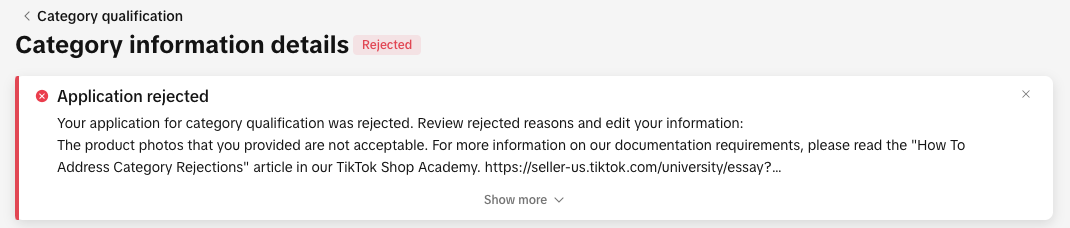
You can view your rejection message by:
- Clicking the bell icon at the top of your Seller Center homepage to go to your inbox
- Opening the rejected application in the Qualification Center.
- Go to the Qualification Center, then click Category Qualification.
- Click the rejected category to view the rejection reason.
- Review the related category policy to confirm what’s needed
- Update or replace your documentation to meet the category’s requirements.
- Click Resubmit to submit your revised application.