Beauty and Personal Care Products Policy
12/15/2025
This policy outlines the requirements for selling Beauty and Personal Care Products on TikTok Shop.Key Points:
- To sell Beauty and Personal Care Products, you may need to submit documentation through the Qualification Center. Requirements vary depending on your role as a seller.
- For help with the review process and rejection handling, see Your Guide to Category Qualification.
Beauty and Personal Care Products
Beauty and personal care products are designed for cleansing, beautifying, and supporting the hygiene and well-being of skin and hair. These include items formulated for babies, children, and adults, such as:- Baby wipes
- Baby powder
- Diaper creams
- Children’s toothpaste
- Body wash and shampoos
- Lotions and creams
- Nail polish
- Mascaras
- Lipsticks
- Foundations
- Hair dyes
- Perfumes
- Perfume decants—repackaged portions of branded fragrances
- Body sunscreen
- Facial sunscreen
- Deodorants
- Acne treatment gel
Requirements To Sell Beauty and Personal Care Products
You may be required to pass a category qualification process to sell Beauty and Personal Care Products on our platform. This process involves submitting documentation that shows you are qualified to sell in this category.Detailed requirements are outlined in collapsible sections below. Please expand each section to view the necessary information.
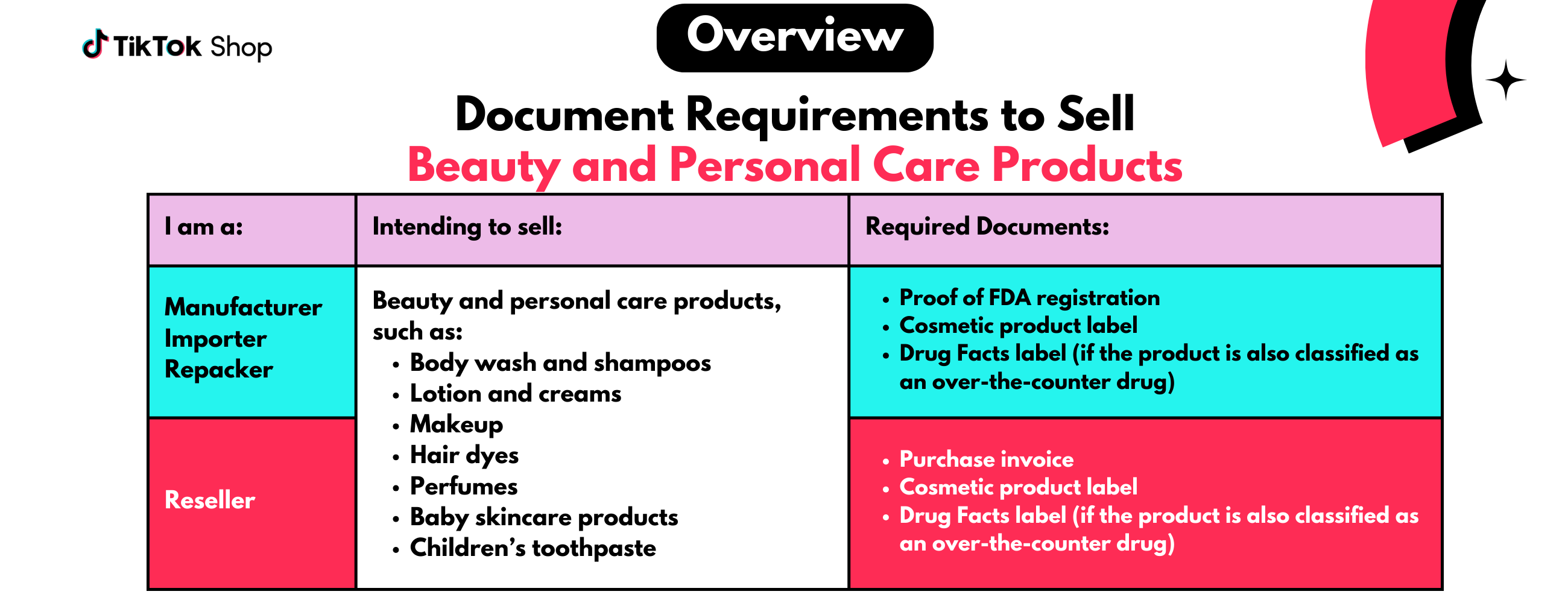
Manufacturers, Importers, And Repackers
If you are applying to sell Beauty and Personal Care Products you manufactured, imported, or repacked, you may be required to submit the following documents:
 Manufacturers, importers, or repackers may be required to provide proof of FDA registration, which must include the following:
Manufacturers, importers, or repackers may be required to provide proof of FDA registration, which must include the following:
 Manufacturers, importers or repackers may be required to submit a cosmetic product label, which must include the following:
Manufacturers, importers or repackers may be required to submit a cosmetic product label, which must include the following:
 Manufacturers, importers, or repackers may be required to submit a drug facts label if the product offers health-related or medical benefits (OTC drug). The drug facts label must include:
Manufacturers, importers, or repackers may be required to submit a drug facts label if the product offers health-related or medical benefits (OTC drug). The drug facts label must include:
- Proof of FDA Registration

- Name of establishment. The name must match with the manufacturer, importer, or repacker as indicated on the product label
- Physical address of establishment
- Operation type of the registered establishment
- Registration number
- The FDA registration must be active at the time of submission
- FDA registration verification letter issued by a third-party
- If the FDA registration verification letter is issued by a third party, the third party name and contact information must be clearly identified on the verification letter.
- Screenshot of FDA registration portal
- If a screenshot of FDA registration is submitted, the image must be able to indicate that the screenshot is from the FDA website.
- If the seller qualifies for a MoCRA registration exemption (e.g., small businesses), they must provide self-attested documentation confirming their exemption status.
- This document should clearly state that the seller meets the criteria for exemption and is self-attesting to that eligibility.
- For more information, refer to the MoCRA Exemption Guidance.
- Cosmetic Product Label

- Principal display panel
- Product name
- Net contents
- Information panel
- Ingredient list
- Manufacturer, importer, or distributor's name and address
- Warning statements
- Drug Facts Label

- Product name
- Net contents
- Drug facts panel
- Active ingredients
- Inactive ingredients
- Direction or use instruction
- Warning statements
- Expiration date
- The expiration date must be presented with month, date, and year OR month and year. The month can be represented as numbers, a full word, or an abbreviation.
- Other information
Resellers
If you are applying to sell Beauty and Personal Care Products as a reseller, you may be required to submit the following documents:
 Resellers may be required to submit a purchase invoice, which must include the following:
Resellers may be required to submit a purchase invoice, which must include the following:
 Resellers may be required to submit a cosmetic product label, which must include the following:
Resellers may be required to submit a cosmetic product label, which must include the following:
 Resellers may be required to submit a drug facts label if the product offers health-related or medical benefits (OTC drug). The drug facts label must include:
Resellers may be required to submit a drug facts label if the product offers health-related or medical benefits (OTC drug). The drug facts label must include:
- Purchase Invoice

- Supplier's company name and address.
- Invoice must be issued within 365 days.
- The product shown on the invoice must match the product category that will be listed on the TikTok Shop.
- The invoice must be in English.
- Cosmetic Product Label

- Principal display panel
- Product name
- Net contents
- Information panel
- Ingredient list
- Manufacturer, importer, or distributor's name and address
- Warning statements
- Drug Facts Label

- Product name
- Net contents
- Drug facts panel
- Active ingredients
- Inactive ingredients
- Direction or use instruction
- Warning statements
- Expiration date
- The expiration date must be presented with month, date, and year OR month and year. The month can be represented as numbers, a full word, or an abbreviation.
- Other information
Prohibited Products
The following beauty and personal care products are prohibited for sale on TikTok Shop:- Beauty and personal care products that claim to have medical applications but are not verified by the United States Food and Drug Administration (FDA).
- Beauty and personal care products that are not labeled correctly as both a cosmetic and a drug.
- Products which aim to bleach, whiten, lighten or reduce overall melanin in the skin are strictly prohibited. However, products designed to enhance the complexion's brightness, even skin tone or reduce dark spots are permissible
- Beauty and personal care products that are not packaged, labeled, or intended for retail sale in the U.S. (for example, cosmetic testers, repackaged cosmetics, imported cosmetics that do not comply with U.S. laws and regulations).
- Cosmetics, beauty, and personal care products that are intended for use by medical professionals or under medical supervision.
- Baby products that have not been approved by the FDA.
- All cross-border products intended for the use of children under the age of three years old.
- Cosmetics that are named in the FDA recall or safety alert
- Cosmetics containing FDA-prohibited or restricted ingredients
Additional Compliance Requirements
Cosmetic products may be sold on TikTok Shop if they comply with all applicable laws and regulations, including but not limited to:
For more information, see Modernization of Cosmetics Regulation Act of 2022 (MoCRA).
- FDA regulations related to cosmetic safety
- Federal Trade Commission (FTC) Fair Packaging and Labeling Act
- Applicable state regulations
- Adverse Event Reporting
- Good Manufacturing Practice (GMP) Manufacturing
For more information, see Modernization of Cosmetics Regulation Act of 2022 (MoCRA).
- Facility Registration and Product Listings
- Registering facilities
- Listing marketed products
- Updating submissions as required
- Safety Substantiation
- Maintain records demonstrating that each cosmetic product is safe for its intended use
- Provide safety evidence. This may include tests, studies, research, analyses, or other information reviewed by qualified experts
Enforcement Actions and Appeals
We regularly review your shop’s compliance with this policy. If any violations are identified, TikTok Shop may take enforcement action at our sole discretion. This may include, but is not limited to:- Rejecting your category qualification application
- Deducting points from your account health
- Removing product listings
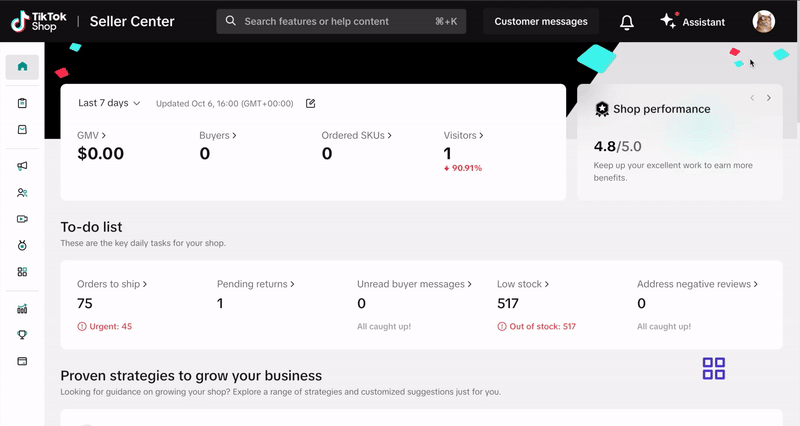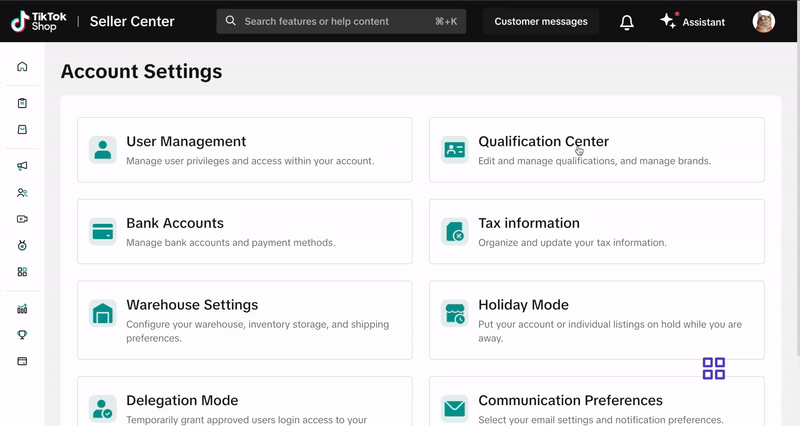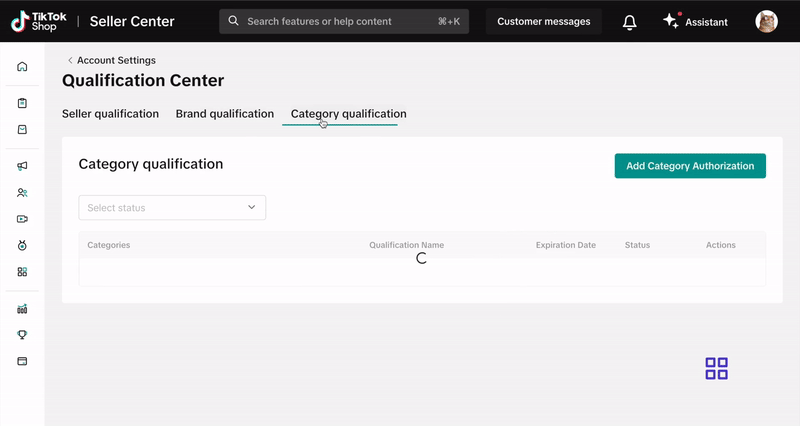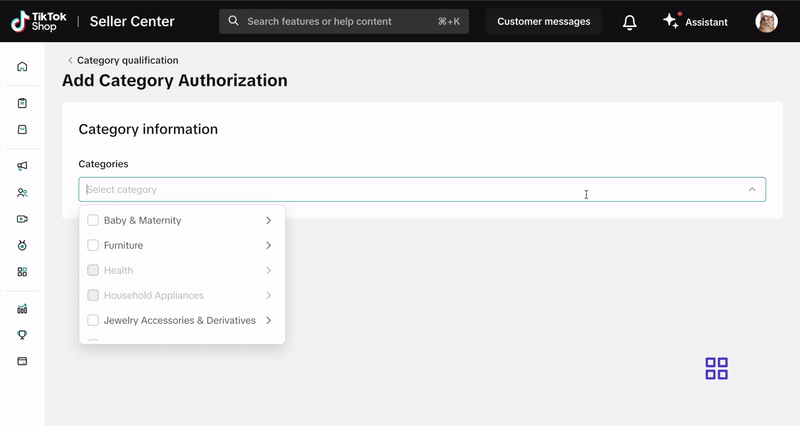
How to Submit Your Documentation
Submit your documentation via the Qualification Center in Seller Center. Click here or follow these steps:- Log into your Seller Center account.
- Click your shop icon in the top right corner.
- Go to My Account > Account Settings.
- Select Qualification Center.
- Click on Category Qualification.
- Click Add Category Authorization and follow the prompts to submit your application.
How To Address A Category Qualification Rejection
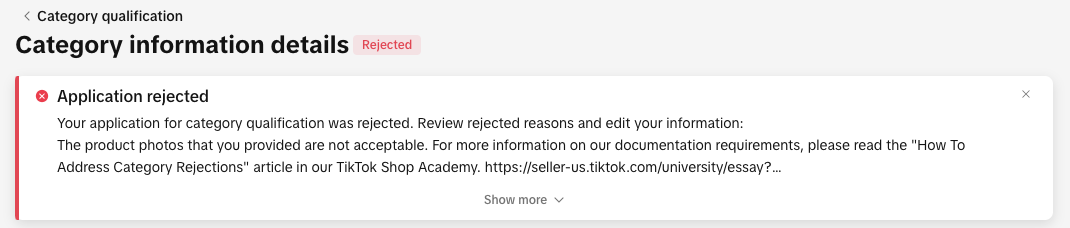
You can view your rejection message by:
- Clicking the bell icon at the top of your Seller Center homepage to go to your inbox
- Opening the rejected application in the Qualification Center.
- Go to the Qualification Center, then click Category Qualification.
- Click the rejected category to view the rejection reason.
- Review the related category policy to confirm what’s needed
- Update or replace your documentation to meet the category’s requirements.
- Click Resubmit to submit your revised application.