Your Guide to Category Qualification
11/11/2025

Some products on TikTok Shop need approval before listing. This process is called category qualification. It ensures that high-risk or regulated products meet TikTok Shop’s standards.This article directs you to the right category policy. There, you can check which documents are required and what to review before reapplying.Key Points:
- Some product types need category qualification before they can be listed.
- This article won’t fix every rejection. It does share application requirements and where to find the right policy.
- Many categories ask for similar documents. Check what’s commonly required before applying!
Which Products Need Category Qualification?
Products that require category qualification include:- Automotive Parts
- Baby and Maternity Products
- Beauty and Personal Care
- Dietary Supplements
- Electronics
- Food and Beverage (Shelf-Stable)
- Household Appliances
- Kids’ Fashion
- Live Plants
- Medical Devices and Supplies
- Pet Supplies
- Sports and Outdoor Recreation
- Toys and Hobbies
How to Apply
Before applying, review the policy for the type of product you want to sell. Gather all required documents before you submit your application. Applications submitted with missing or incorrect documents will be rejected.To submit your application:Tip: If you don’t have the documents, contact your supplier or manufacturer. They’re usually required to provide them.
- Log in to your Seller Center account.
- Click your shop icon in the top-right corner.
- Go to My Account > Account Settings.
- Select Qualification Center, then click Category Qualification.
- Click Add Category Authorization. Follow the prompts to upload your documentation and submit your application.
If Your Application Is Rejected
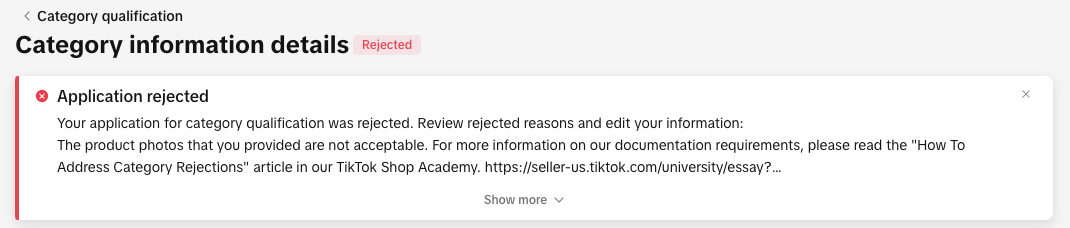
You can view your rejection message by:
- Clicking the bell icon at the top of your Seller Center homepage to go to your inbox
- Opening the rejected application in the Qualification Center.
- Go to the Qualification Center, then click Category Qualification.
- Click the rejected category to view the rejection reason.
- Review the related category policy to confirm what’s needed
- Update or replace your documentation to meet the category’s requirements.
- Click Resubmit to submit your revised application.
Common Documentation
Most category qualification rejections occur when sellers submit incomplete, incorrect, or low-quality documents. Some document types are commonly required across multiple product categories.Below are examples of documentation that may be required. Always check the category policy linked above to confirm what’s needed for your product.Product Photos 📸

- The full product and its packaging
- Label information, including ingredients, directions, and net content
- Manufacturer or distributor's name and address
- Any required safety markings (for electronics) or expiration dates (for perishable products)
Photos must be clear, unedited, and show the actual product being sold.
Purchase Invoice 🧾
Make sure it includes:
- Supplier name and contact info
- Purchase date (within the past 12 months)
- Product details that match your listing
Packing slips, marketplace orders, or screenshots are usually not accepted.
FDA Documentation 🏛️
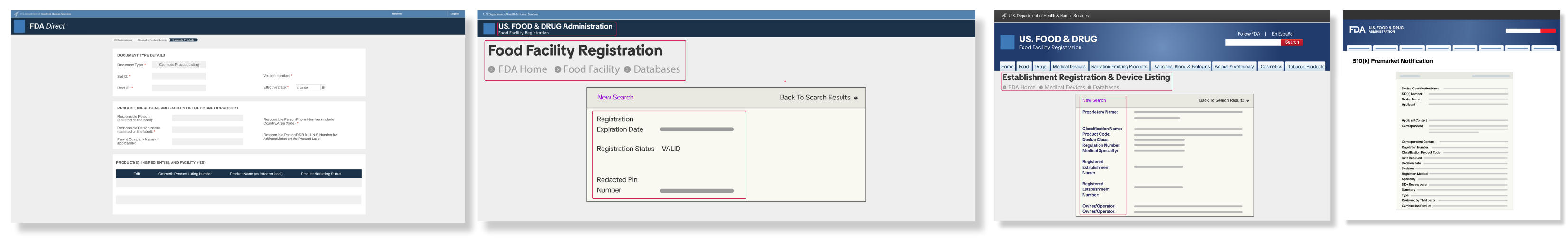 For beauty, personal care, food, beverage, and medical products, FDA registration or other supporting documentation may be required. Examples include:
For beauty, personal care, food, beverage, and medical products, FDA registration or other supporting documentation may be required. Examples include:- Cosmetic Product Listing Number (CPLN) for general cosmetics
- National Drug Code (NDC) for OTC drugs or medicated cosmetics
- 510(k) Premarket Notification or Exemption for certain medical devices
- Device Establishment Registration for FDA-regulated tools like facial devices or whitening kits
Make sure any documents submitted are official, legible, and unaltered.
Certificate of Compliance ✅

- The name of the manufacturer or importer
- The product type and model
- The standards or testing performed (e.g. UL or FCC)
- A valid issue or expiration date (typically within 5 years)
Frequently Asked Questions
How long does it take for TikTok Shop to review my application?
Most applications are reviewed within six days. Processing times may vary depending on review volume.
What should I do if I’m unsure whether my documents meet the requirements?
Start by reviewing the policy for the type of product you want to sell. If you’re still unsure, contact your product manufacturer or supplier for help.
Can I submit multiple applications for the same category at the same time?
No. You can only have one active application per category. If your application is under review, wait for the decision before submitting another.