Documents Required to Sell Medical Devices and Medical Supplies
07/30/2025

How to Sell Medical Devices on TikTok Shop
Medical Devices are classified as restricted products on TikTok Shop. To sell them, you will need to go through TikTok Shop's Category Qualification process, which requires submitting necessary documentation.Only sellers who pass Category Qualification can sell medical devices on TikTok Shop. Following these guidelines will help you successfully complete this process and ensure a smooth selling experience.
Classification of Medical Devices
Medical devices are FDA-regulated and classified by intended use. To determine if your product qualifies as a medical device and to identify its classification, click here. Class III medical devices are prohibited on TikTok Shop.The following categories may have medical devices:
- Baby & Maternity
These products require additional documentation, including a Children’s Product Certificate (CPC), a test report from a Consumer Product Safety Commission (CPSC) accredited laboratory, and a sample tracking label. For more information, refer to the Documents Required to Sell Baby & Maternity Products.
- Beauty & Personal Care
- Fashion Accessories
- Health
- Certain high-risk health products are only permitted on TikTok Shop through an invite-only process, including glucose monitors, electric and manual blood pressure monitors, cholesterol monitors, glucose control solutions, and glucose kits.
- Sports & Outdoor
Document Requirements for Medical Devices
If you are a manufacturer or importer:- Proof of FDA Establishment Registration & Device Listing
- 510(K) Pre-Market Notification or Proof of Exemption (Class II only)
- Product label image
- If the medical device is electrical:
- Certification or registration of Compliance / Conformity (COC)
- Image of valid Product Electrical Safety Marking
- Image of valid Product FCC Safety Marking (if the product has Bluetooth or Wi-Fi)
- Purchase invoice
- Product label image
- If the medical device is electrical:
- Image of valid Product Electrical Safety Marking
- Image of valid Product FCC Safety Marking (if the product has Bluetooth or Wi-Fi)
Proof of FDA Establishment Registration & Device Listing
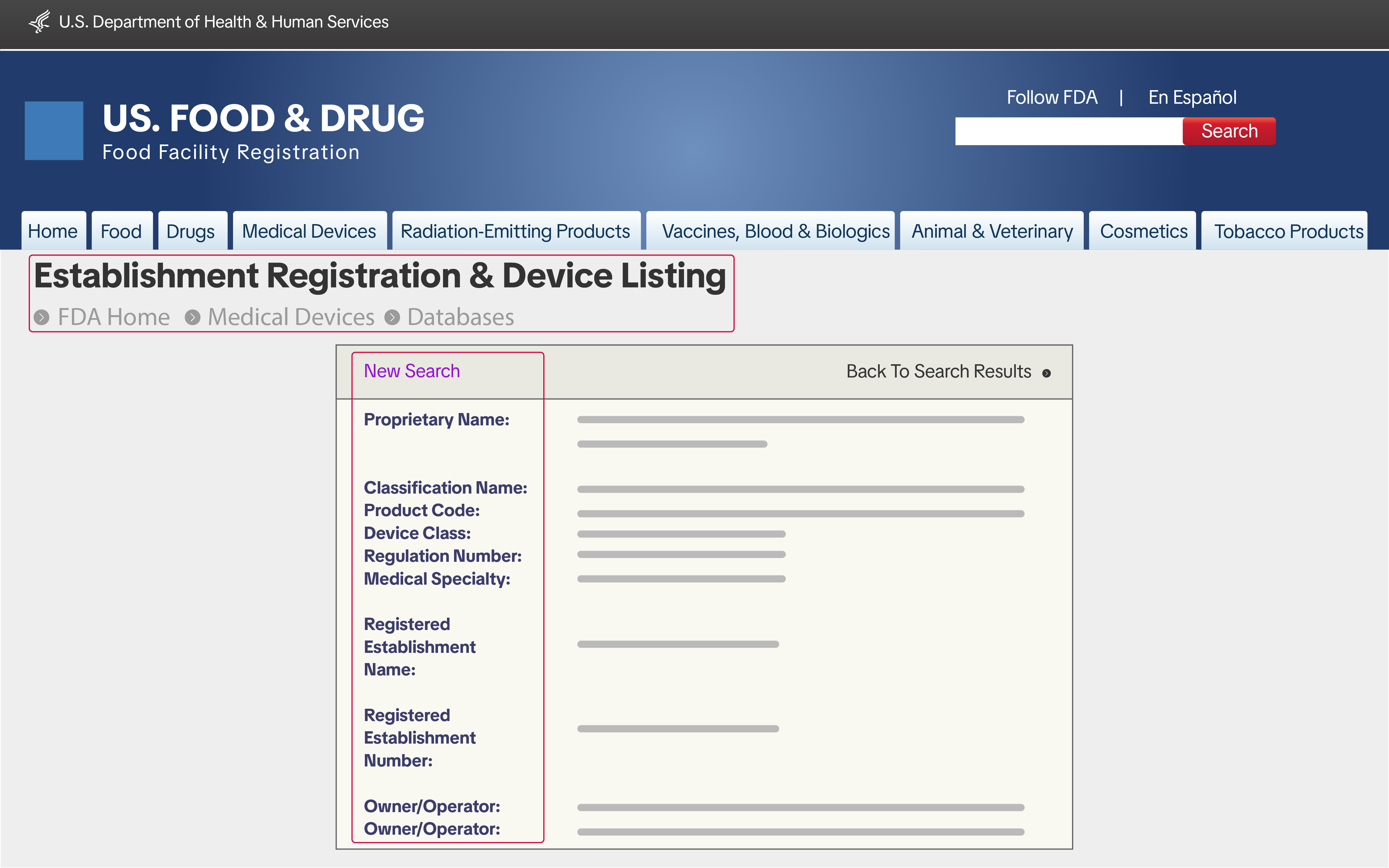 Manufacturers and importers selling Class I and II medical devices on TikTok Shop must submit proof of FDA Establishment Registration and Device Listing. You can get this information from the FDA's Unified Registration and Listing System (FURLS).
Manufacturers and importers selling Class I and II medical devices on TikTok Shop must submit proof of FDA Establishment Registration and Device Listing. You can get this information from the FDA's Unified Registration and Listing System (FURLS).510(K) Pre-Market Notification or Proof of Exemption (Class II only)
 Manufacturers and importers selling Class II medical devices on TikTok Shop must submit a copy of a 510(k) Pre-Market Notification or proof of 510(k) exemption from FDA’s Product Classification database. The following must be included:
Manufacturers and importers selling Class II medical devices on TikTok Shop must submit a copy of a 510(k) Pre-Market Notification or proof of 510(k) exemption from FDA’s Product Classification database. The following must be included:- Manufacturer or importer's name
- The product shown on the copy must match the product category that will be listed on the TikTok Shop, as well as include product description and details.
Purchase Invoice
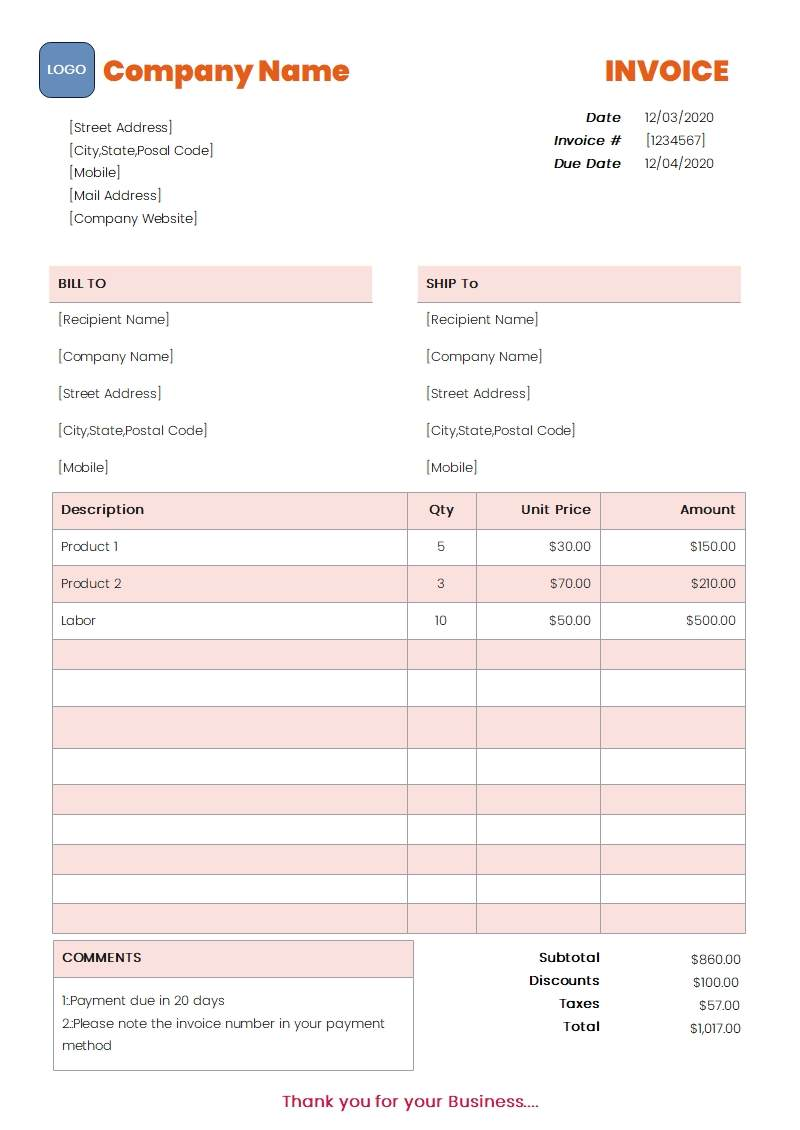 Resellers must provide a purchase invoice issued by a US-based supplier. The purchase invoice must be legible and include the following information:
Resellers must provide a purchase invoice issued by a US-based supplier. The purchase invoice must be legible and include the following information:- Supplier's company name and address
- Invoice must be issued within 365 days
- The product shown on the invoice must match the product category that will be listed on the TikTok Shop
- Invoice must be in English
Product Label Images
 Manufacturers, importers, and resellers must provide an image of the product or packaging.
Manufacturers, importers, and resellers must provide an image of the product or packaging.- The images must show all sides of the product and its packaging. The images should be clear, legible, complete, unedited and in color. Ensure they are at least 600x600 pixels in resolution. Avoid uploading black-and-white, angled, blurry, cropped, or edited images.
- Any information on the product and packaging MUST be visible and legible, including the product description, warning logos, and other information.
Additional Requirements
The requirements listed below are in addition to the document requirements listed in 1-4.Electrical Medical Devices

- If the medical device is electrical and you are a manufacturer or importer:
- A valid Certification of Compliance/Conformity (COC) accredited by the Occupational Safety and Health Administration (OSHA). Ensure it includes the following:
- Contains a clear description and details of the product which has been tested.
- Matches the product you are listing.
- Lists all safety standard tests performed, with clear Pass/Fail indicators.
- Image of valid Product Electrical Safety Marking
- Image of valid Product FCC Safety Marking (if the product has Bluetooth or Wi-Fi)
- A valid Certification of Compliance/Conformity (COC) accredited by the Occupational Safety and Health Administration (OSHA). Ensure it includes the following:
- If the medical device is electrical and you are a reseller:
- Image of valid Product Electrical Safety Marking
- Image of valid Product FCC Safety Marking (if the product has Bluetooth or Wi-Fi)
Surgical N95 Respirators
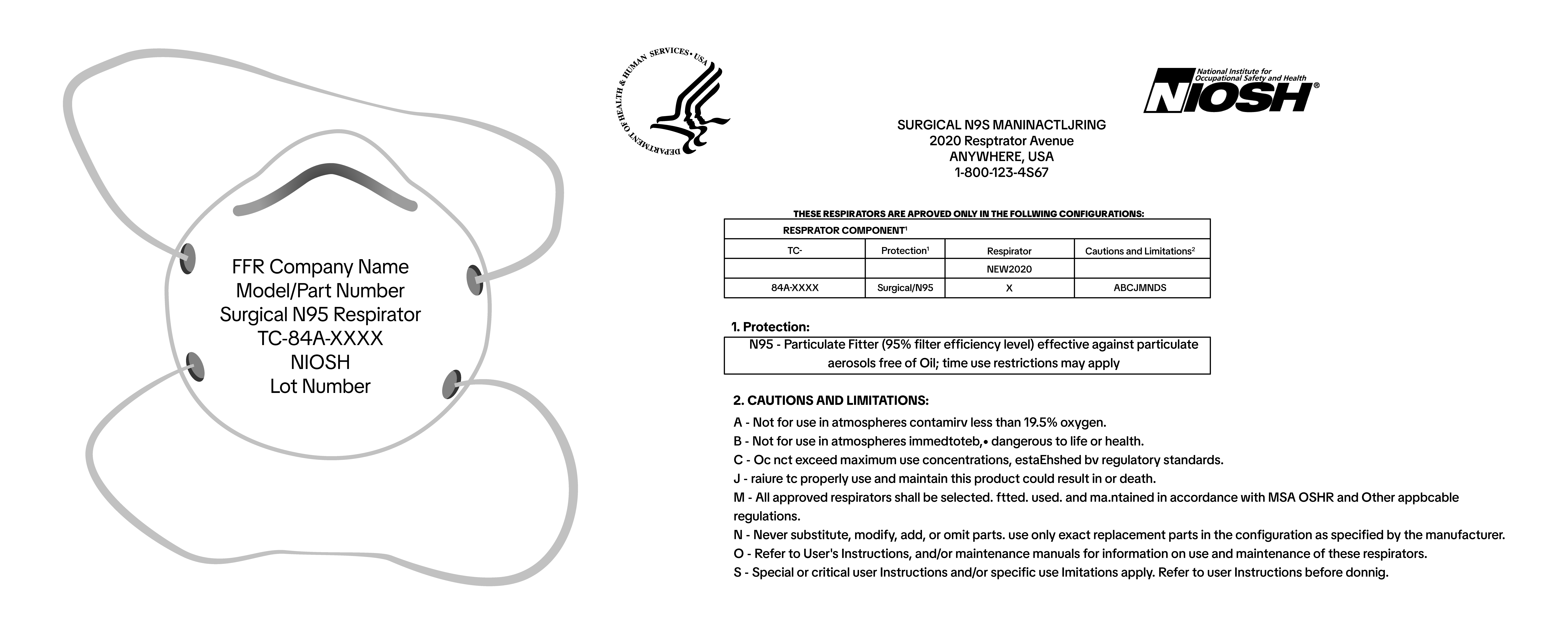 Surgical N95 respirators are Class II medical devices and require additional labeling requirements. This includes:
Surgical N95 respirators are Class II medical devices and require additional labeling requirements. This includes:- Product Label on mask
- Approval Holder / Company name
- Model or Part #
- Surgical N95 Respirator
- TC-Approval Number
- NIOSH (in capital block letters)
- Lot #
- Packaging Label
- Expiration date / Use by Date
- Packaging Label and/or Insert
- NIOSH surgical N95 full respirator approval label, often located on or within the packaging
PPE Masks (Medical masks with liquid barrier protection)
 PPE masks are Class II medical devices that require additional labeling requirements. This includes:
PPE masks are Class II medical devices that require additional labeling requirements. This includes:- Packaging Label
- ASTM F2100 or EN 14683 on the box label
- Expiration date / Use by Date
Ear Drops, Eye Drops, Contact Lens Solutions, and Contact Lens Conditioning Kits
Due to safety and compliance considerations, only select qualified sellers are permitted to offer ear drops, eye drops, contact lens solutions, and contact lens conditioning kits on TikTok Shop. These products require additional oversight and must meet the document requirements outlined below.In addition to the documents listed in sections 1-4, manufacturers, importers, and resellers must submit:
- Certificate of Analysis (COA):
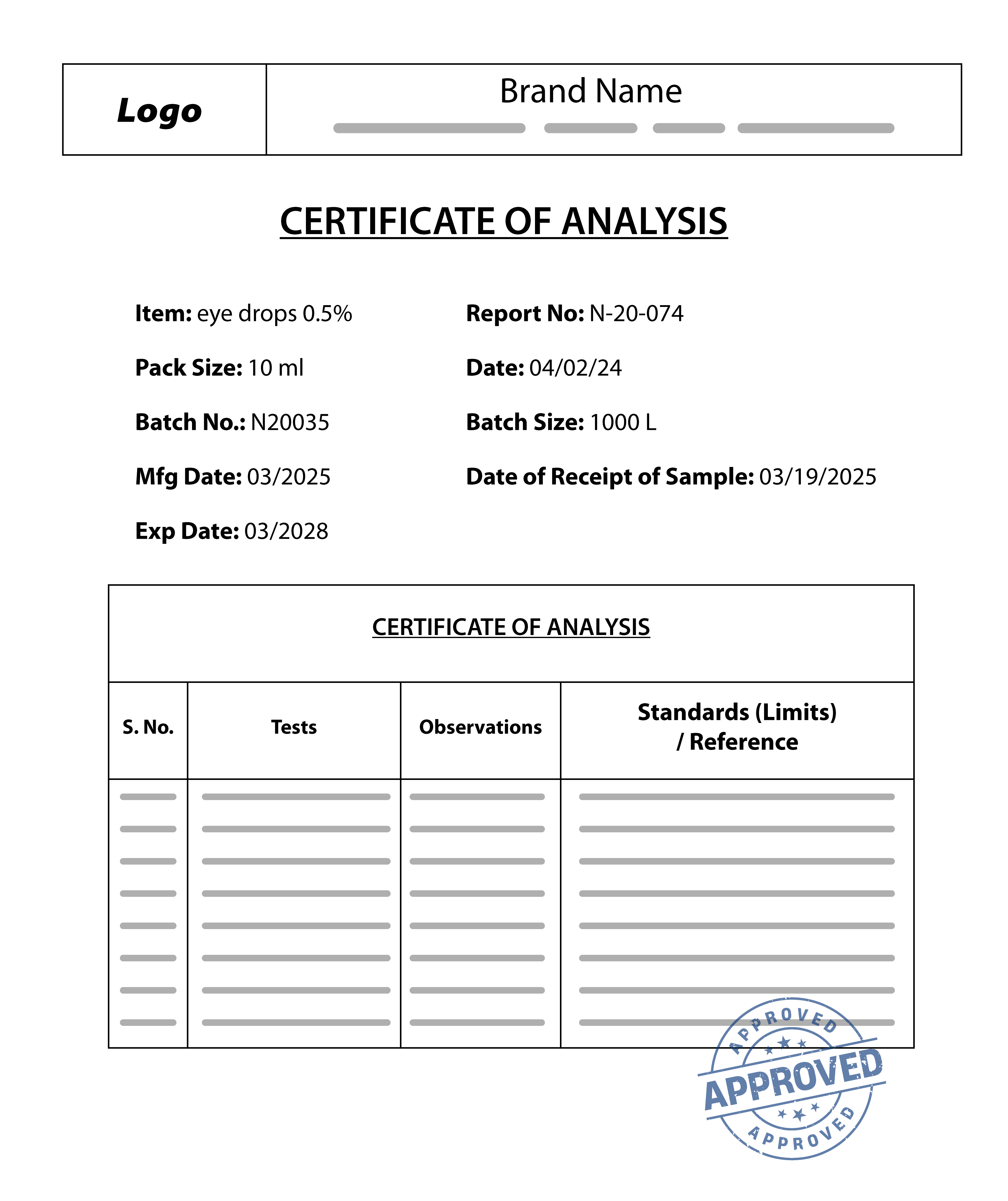
A COA is required to verify your product’s quality, safety, and compliance with regulatory standards. When submitting your COA, ensure it is clear, unaltered, and meets all of the following criteria:
- Contains a clear description and relevant details of the specific product that was tested
- Was issued within the past 2 years
- Matches the product you are listing
- Lists all safety standards tested
- Includes the name and address of the accredited testing laboratory
- Is provided in English
Prohibited Products
The following medical devices and medical supply products are prohibited from sale on TikTok Shop:- Class III medical devices
- Products requiring a prescription written by a healthcare provider
How to Submit Your Documents
You can submit the required documents through the Qualification Center in your Seller Center by clicking here or following the steps below:Desktop:
- Log into your TikTok Seller Center account.
- Click on your shop icon at the top right side of the screen.
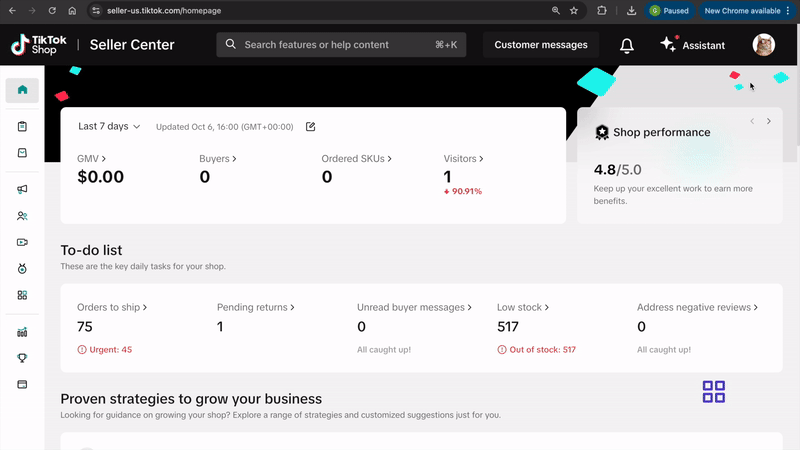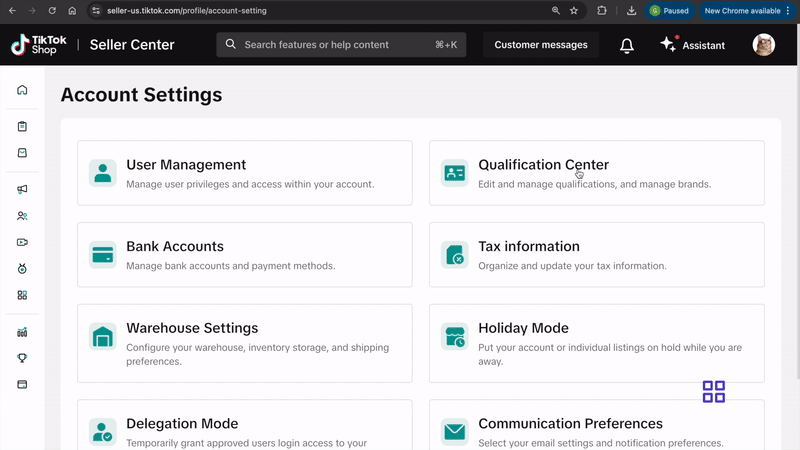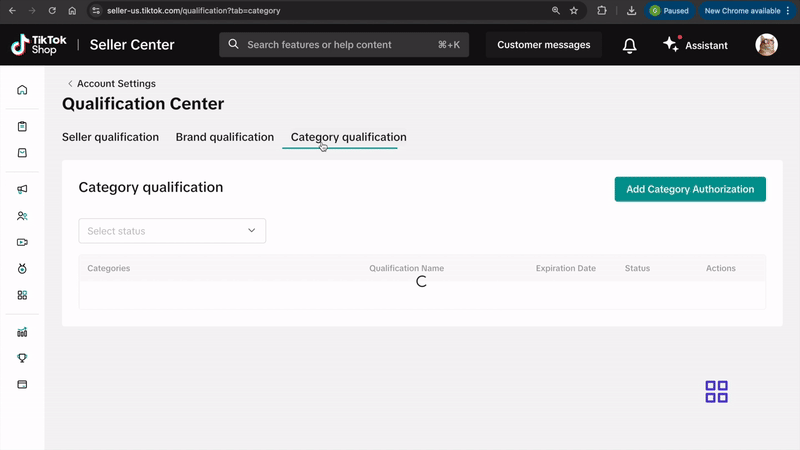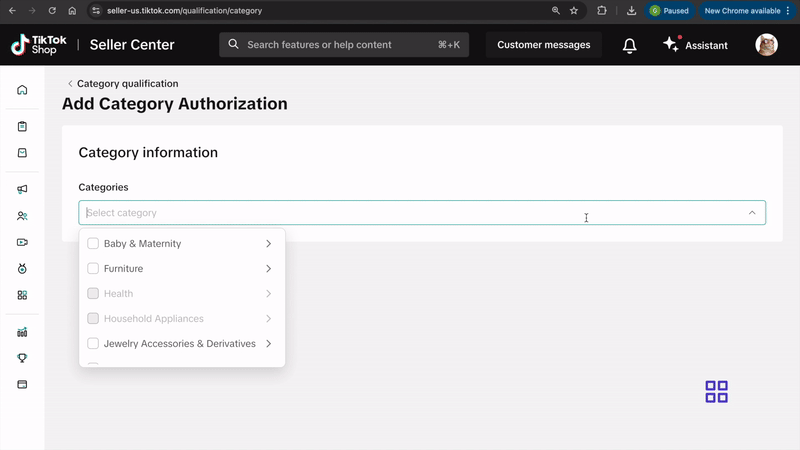
- Go to My Account > Account Settings.
- Select Qualification Center.
- Click on Category Qualification.
- Click on Add Category Authorization and follow the prompts to submit your application.
 Products that claim to be safe for medical or electrical use but lack verification by the US FDA, relevant electrical or FCC testing laboratories, or are inaccurately or fraudulently labeled, violate the TikTok Shop Restricted Products Policy.
Products that claim to be safe for medical or electrical use but lack verification by the US FDA, relevant electrical or FCC testing laboratories, or are inaccurately or fraudulently labeled, violate the TikTok Shop Restricted Products Policy.