Documents Required to Sell Baby and Maternity Products
09/09/2025

How to Sell Baby & Maternity Products on TikTok Shop
Baby and maternity products are classified as restricted products on TikTok Shop. To sell them, you will need to go through TikTok Shop's Category Qualification process, which requires submitting the necessary documentation.Only sellers who pass Category Qualification can sell baby and maternity products on TikTok Shop. Following these guidelines will help you successfully complete this process and ensure a smooth selling experience.
This guide outlines the document requirements for selling products in these categories:
- Baby and maternity products
- Baby and maternity medical devices: Class I and Class II
- Baby and maternity sterilizers
- Baby skincare products
- Electronic children's products
Exempted Products
Some items within the baby and maternity products category do not require certification, as they are not primarily designed or intended for children 12 years of age or younger.Baby & Maternity Products
 Baby and maternity products encompass a wide variety of items that cater to the needs of infants and toddlers up to three years old, as well as expecting and new mothers. Examples include:
Baby and maternity products encompass a wide variety of items that cater to the needs of infants and toddlers up to three years old, as well as expecting and new mothers. Examples include:- Baby clothing
- Bath towels, bath thermometers, bathing tubs
- Baby toys, such as playmats, soft toys, and books
- Baby brushes and combs
- Baby bottles and utensils
- Baby car seats
- Baby carriers
- Baby furniture, such as sleep soothers, rugs, cushions, playpens, highchairs, and seats
- Diapers
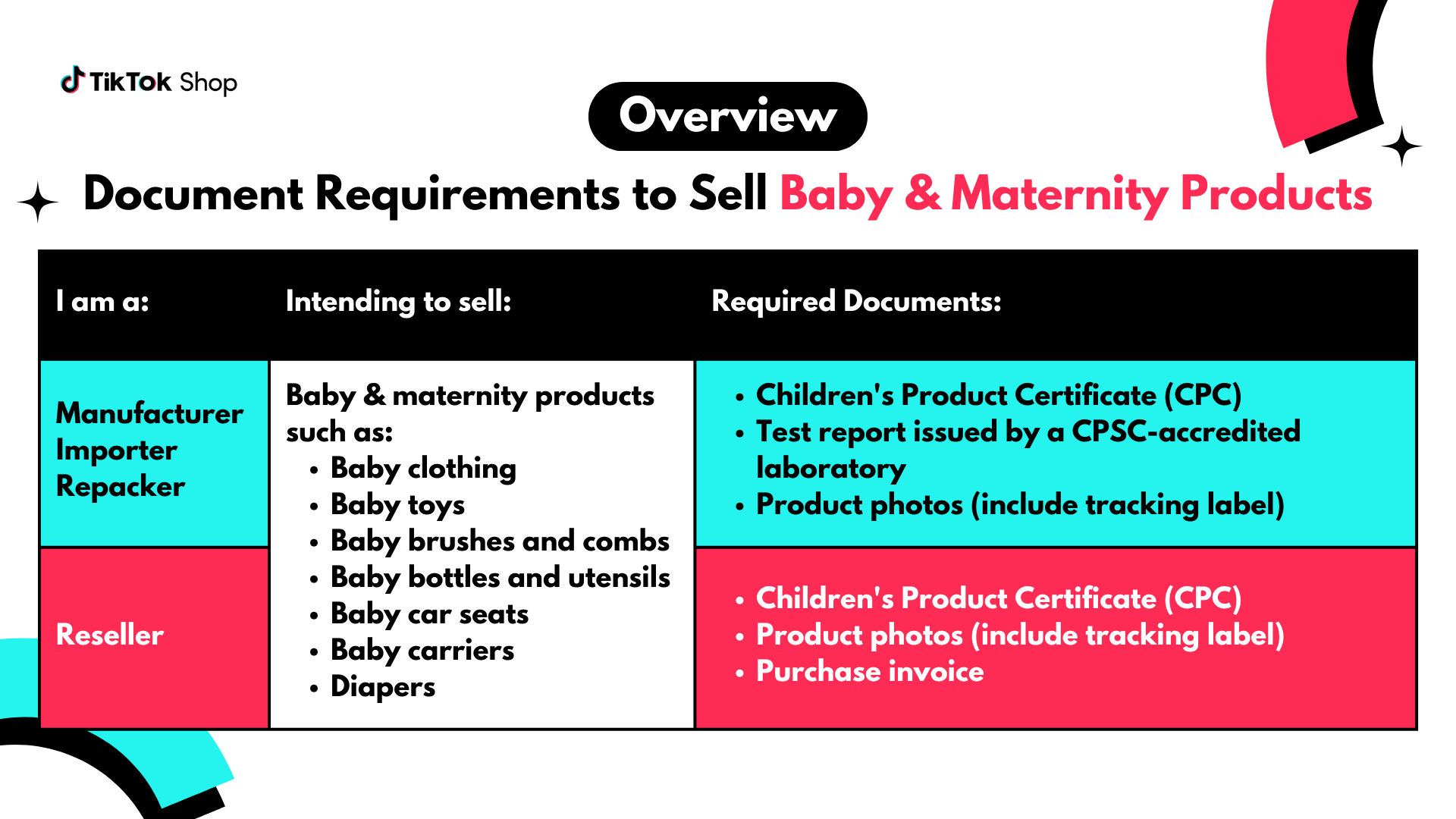
Document Requirements for Baby & Maternity Products
If you are a manufacturer, importer, or repacker, submit these documents:- Children's Product Certificate (CPC)
- Test report issued by a CPSC-accredited laboratory
- Product photos (include tracking label)
- Children's Product Certificate (CPC)
- Product photos (include tracking label)
- Purchase invoice
Baby & Maternity Medical Devices
Baby and maternity medical devices are essential tools for infants and maternal care and are classified as either Class I or Class II.- Class I devices are simpler products that require less extensive testing. Examples include:
- Manual toothbrushes
- Electronic toothbrushes
- Dental care kits
- Baby sunglasses
- Class II devices involve more complex functions and require additional documentation. Examples include:
- Nasal aspirators
- Ear syringe sets
- Breast pumps and accessories
- Teethers, soothers, dummies, or pacifiers that do not ease teething pain are regulated by the Consumer Product Safety Commission (CPSC). For documentation requirements, please refer to the 'Baby & Maternity Products' section.
- Teethers, soothers, dummies, or pacifiers that ease teething pain fall are regulated by the U.S. Food and Drug Administration (FDA). For documentation requirements, please refer to the 'Class II Baby & Maternity Medical Devices' section.
Document Requirements for Baby & Maternity Medical Devices
Class I Medical Devices
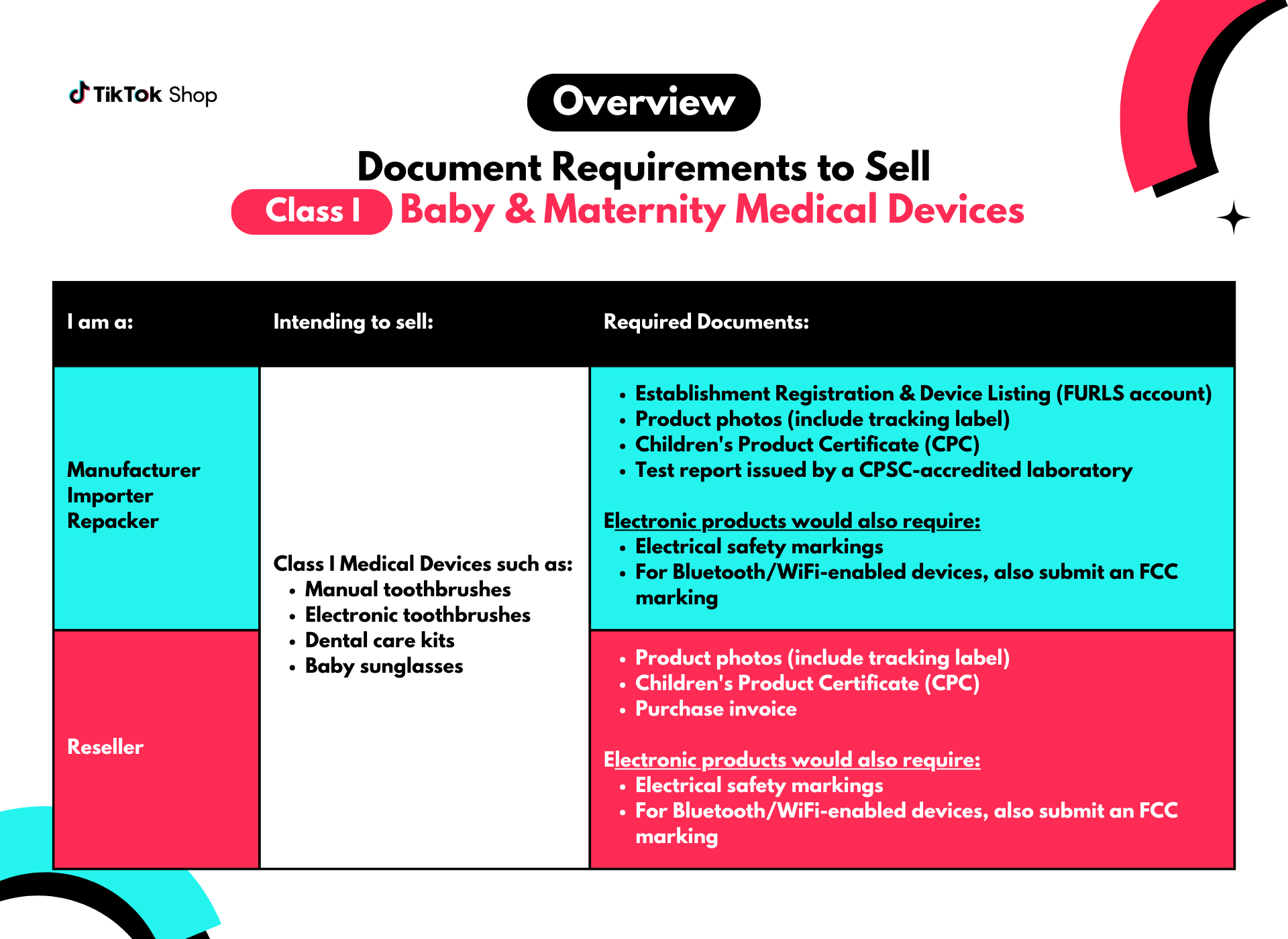 If you are a manufacturer, importer, or repacker, submit these documents:
If you are a manufacturer, importer, or repacker, submit these documents:- Establishment Registration & Device Listing (FURLS account)
- Product photos (include tracking label)
- Children's Product Certificate (CPC)
- Test report issued by a CPSC-accredited laboratory
- Electrical safety markings
- For Bluetooth/WiFi-enabled devices, also submit an FCC marking
- Product photos (include tracking label)
- Children's Product Certificate (CPC)
- Purchase invoice
- Electrical safety markings
- For Bluetooth/WiFi-enabled devices, also submit an FCC marking
Class II Medical Devices
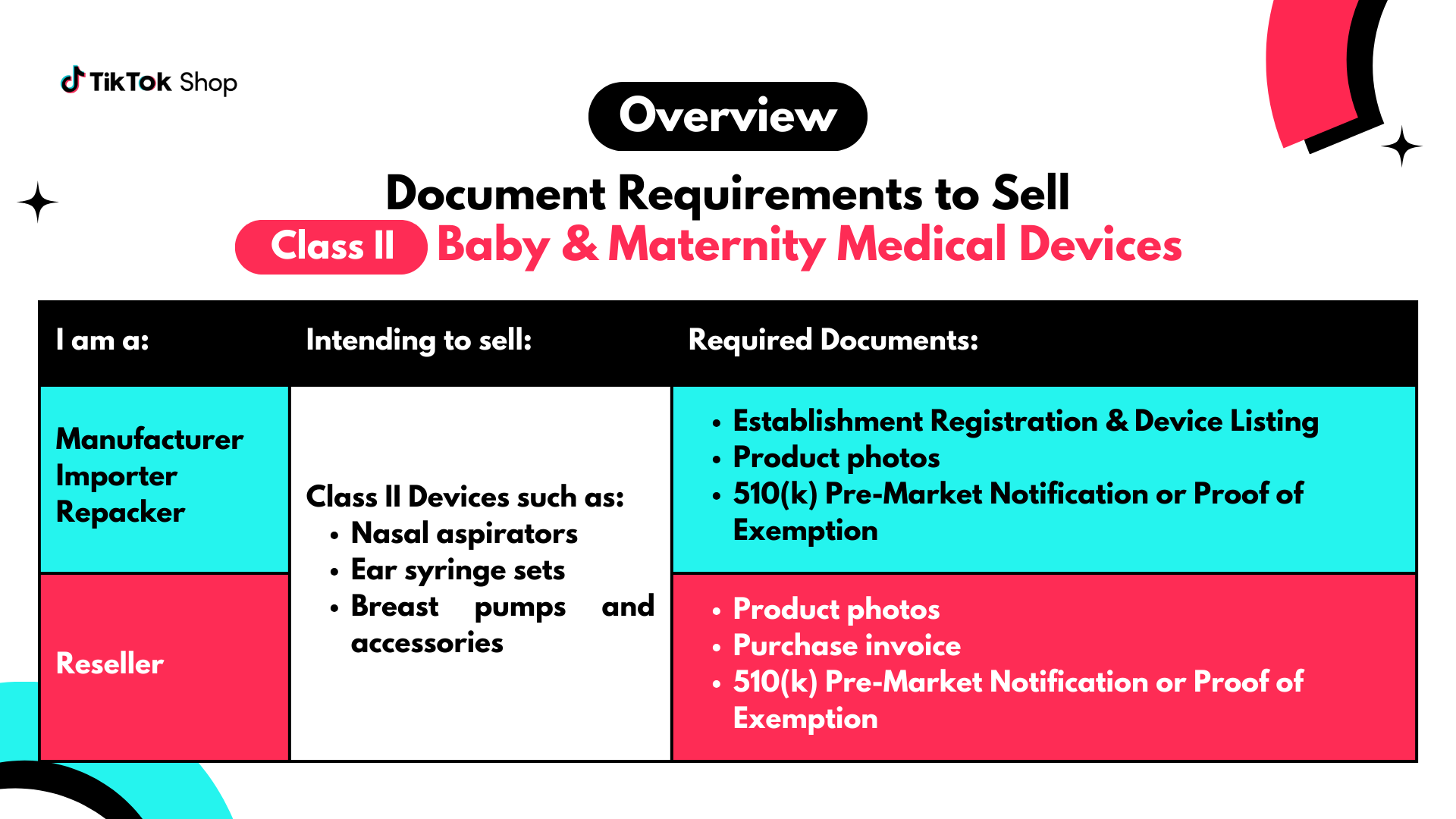 If you are a manufacturer, importer, or repacker, submit these documents:
If you are a manufacturer, importer, or repacker, submit these documents:- Establishment Registration & Device Listing (FURLS account)
- Product photos (include tracking label)
- Children's Product Certificate (CPC)
- Test report issued by a CPSC-accredited laboratory
- 510(k) Pre-Market Notification or Proof of Exemption
- Electrical safety markings
- For Bluetooth/WiFi-enabled devices, also submit an FCC marking
- Product photos (include tracking label)
- Children's Product Certificate (CPC)
- Purchase invoice
- Electrical safety markings
- For Bluetooth/WiFi-enabled devices, also submit an FCC marking
Baby & Maternity Sterilizers
 Baby and maternity sterilizers are essential for maintaining a safe and hygienic environment for infants. To ensure safety and compliance, these products require specific documentation. Example products include:
Baby and maternity sterilizers are essential for maintaining a safe and hygienic environment for infants. To ensure safety and compliance, these products require specific documentation. Example products include:- Baby bottle warmers, coolers, and sterilizers
- Baby clothing sterilizers
Document Requirements for Baby & Maternity Sterilizers
If you are a manufacturer, importer, or repacker, submit these documents:- Product photos
- Include EPA Establishment Number on the label or packaging for sterilizers or products with sterilization claims
- Electrical Safety Markings
- For Bluetooth/WiFi-enabled devices, also submit an FCC marking
- Product photos
- Include EPA Establishment Number on the label or packaging for sterilizers or products with sterilization claims
- Electrical Safety Markings
- For Bluetooth/WiFi-enabled devices, also submit an FCC marking
- Purchase invoice
Baby Skincare Products
 Baby skincare products are specially formulated to protect, nourish, and maintain the well-being of a baby’s skin, hair, and overall hygiene. Examples include:
Baby skincare products are specially formulated to protect, nourish, and maintain the well-being of a baby’s skin, hair, and overall hygiene. Examples include:- Baby Sunscreen
- Baby Oils, Lotions, Creams
- Baby Body Washes, Shampoo, and Conditioner
- Baby Wipes and Wipe Refills
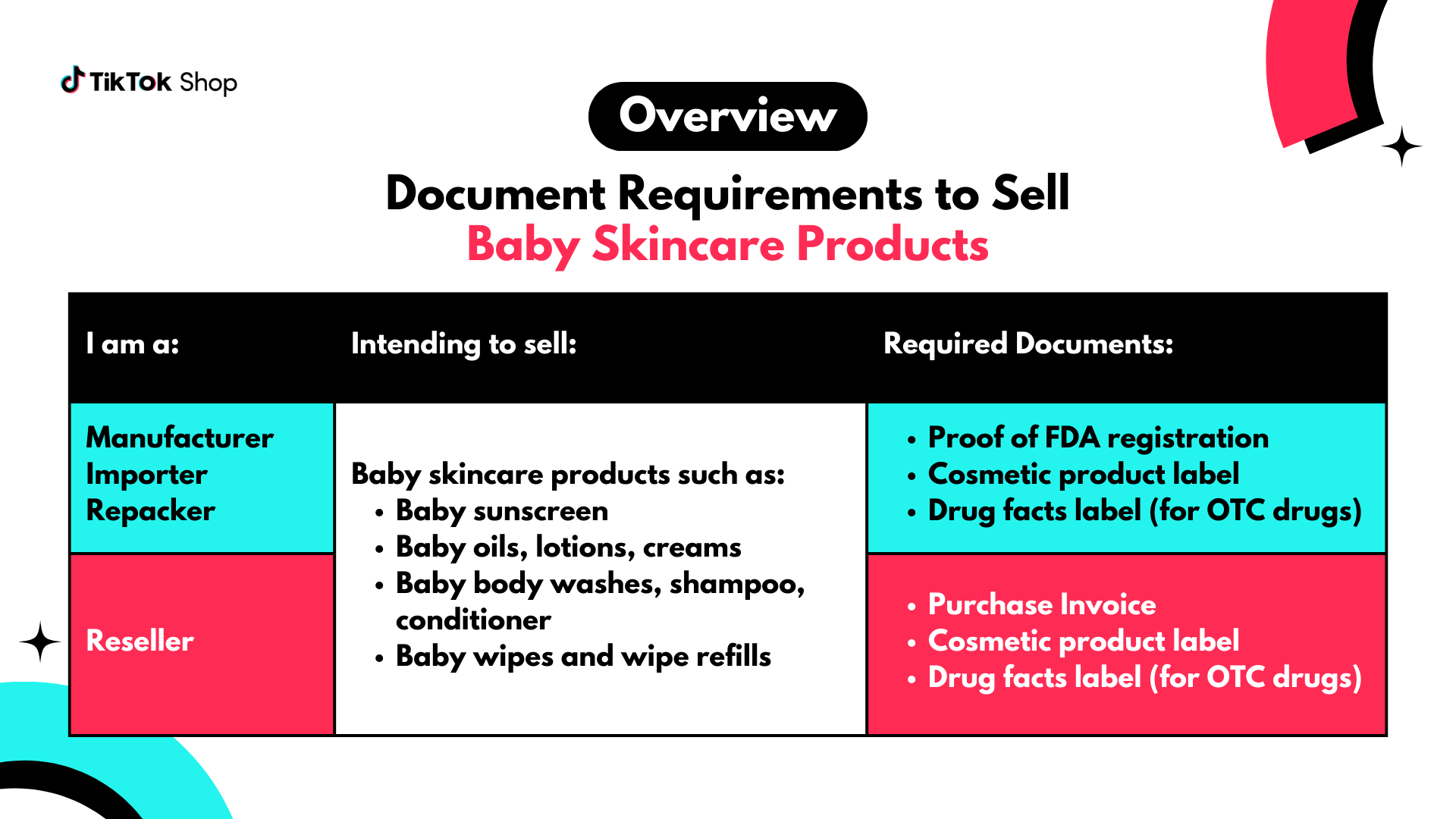
Document Requirements for Baby Skincare Products
If you are a manufacturer, importer, or repacker, submit these documents:- Proof of FDA registration
- Cosmetic Product Label
- Drug Facts Label (if your product is also classified as an over-the-counter drug)
- Purchase invoice
- Cosmetic Product Label
- Drug Facts Label (if your product is also classified as an over-the-counter drug)
Children's Electronic Products
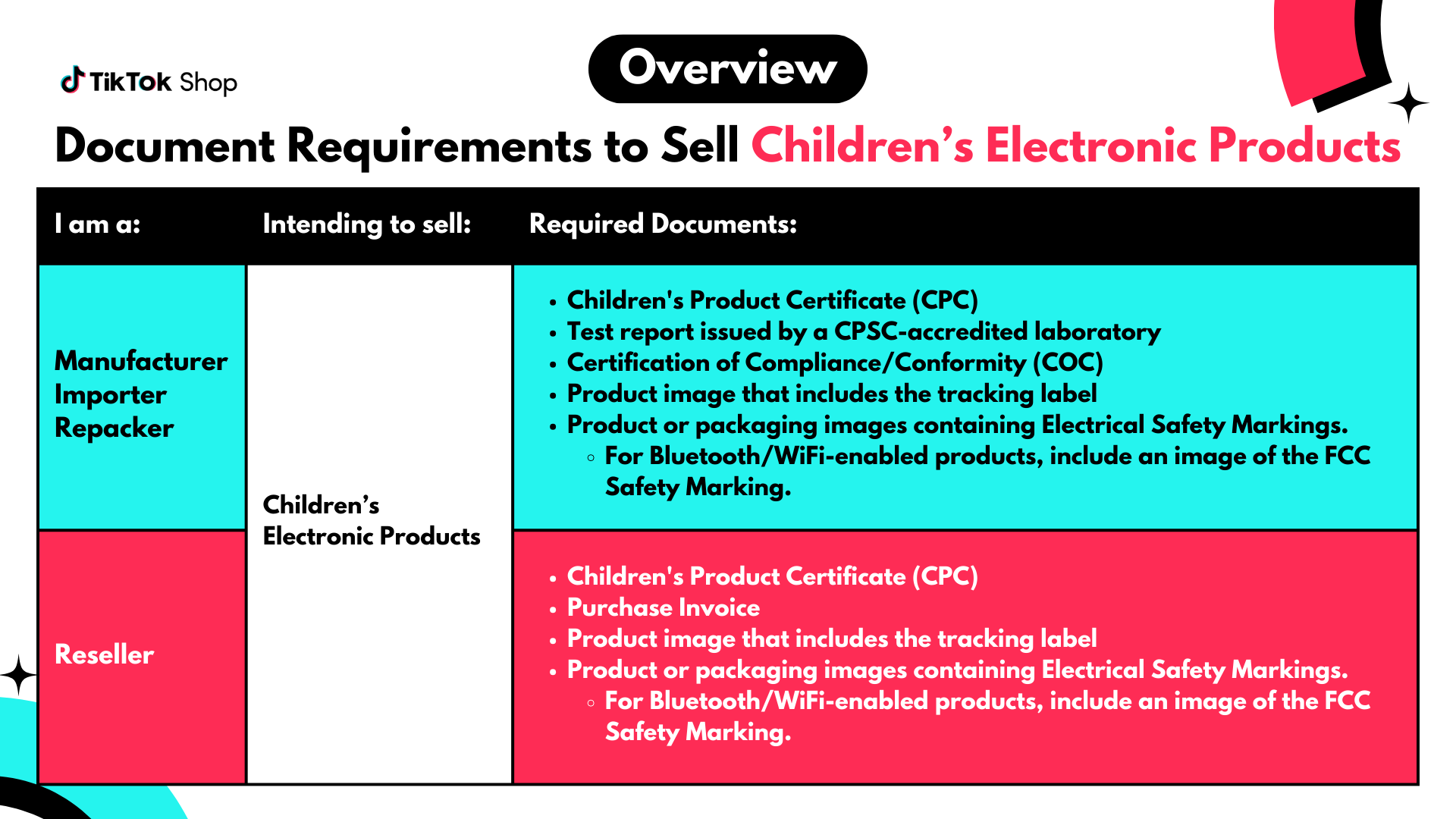
Document Requirements for Children's Electronic Products
If you are a manufacturer, importer, or repacker, submit these documents:- Children's Product Certificate (CPC)
- Test report issued by a CPSC-accredited laboratory
- Certification of Compliance or Conformity (COC)
- Product image that includes the tracking label
- Product or packaging images containing Electrical Safety Markings. For Bluetooth/WiFi-enabled products, also include an image of the FCC Safety Marking.
- Children's Product Certificate (CPC)
- Purchase invoice
- Product image that includes the tracking label
- Product or packaging images containing Electrical Safety Markings. For Bluetooth/WiFi-enabled products, also include an image of the FCC Safety Marking.
Detailed Document Requirements for Baby and Maternity Products
Certification of Compliance or Conformity (COC)
 Manufacturers or importers must provide a Certification of Compliance or Conformity (COC). This must include the following information:
Manufacturers or importers must provide a Certification of Compliance or Conformity (COC). This must include the following information:- Issued within the last 2 years.
- Matches the product category you intend to sell.
- Contains a clear description and details of the product which has been tested.
- Lists all safety standards tested.
- Contains the name and address of the accredited laboratory that conducted the testing.
- Ensure the product described in the report matches the product photos.
Children's Product Certificate (CPC)
 Manufacturers, importers, repackers, and resellers for baby and maternity products and medical devices must submit the Children's Product Certificate (CPC) to verify that their product complies with U.S. consumer product safety standards. Refer to the CPSC’s page on Children’s Product Certificates for more information, including examples and tutorial videos.
Manufacturers, importers, repackers, and resellers for baby and maternity products and medical devices must submit the Children's Product Certificate (CPC) to verify that their product complies with U.S. consumer product safety standards. Refer to the CPSC’s page on Children’s Product Certificates for more information, including examples and tutorial videos.When submitting your CPC, make sure it is legible, free from alterations, and meets the following requirements:
- Include the manufacturer or importer's name and address.
- Issued within the last 365 days.
- Product details must match the product category and photos.
- Include a clear description of the tested product.
- Include the name and address of the CPSP-accredited laboratory.
- List all relevant safety standards tested.
- The CPC should be in English.
Cosmetic Product Label
 Manufacturers, importers, repackers, and resellers must provide a cosmetic product label from a product you intend to sell. The cosmetics label must include:
Manufacturers, importers, repackers, and resellers must provide a cosmetic product label from a product you intend to sell. The cosmetics label must include:- Principal display panel
- Product name
- Net contents
- Information panel
- Ingredient list
- Manufacturer, importer, or distributor's name and address
- Warning statements
Drug Facts Label
 Baby skincare products that offer health-related or medical benefits must provide a drug facts label from a product you intend to sell. The drug facts label must include:
Baby skincare products that offer health-related or medical benefits must provide a drug facts label from a product you intend to sell. The drug facts label must include:- Product name
- Net contents
- Drug facts panel
- Active ingredients
- Inactive ingredients
- Direction or use instruction
- Warning statements
- Expiration date
- The expiration date must be presented with month, date, and year OR month and year. The month can be represented as numbers, a full word, or an abbreviation.
- Other informaton
Electrical Safety Markings
 For electric baby and maternity medical devices, sterilizers, and warmers, manufacturers, importers, repackers, and resellers must provide photos of:
For electric baby and maternity medical devices, sterilizers, and warmers, manufacturers, importers, repackers, and resellers must provide photos of:- A valid electrical safety marking, either as sticker or directly embossed on the product.
- The marking can be printed on the packaging or applied as a sticker.
- Stickers must lay flat with no creases or peeling edges.
- Double stickering is not allowed.
- For Bluetooth/WiFi-enabled devices, also submit an FCC marking.
Establishment Registration & Device Listing (FURLS account)
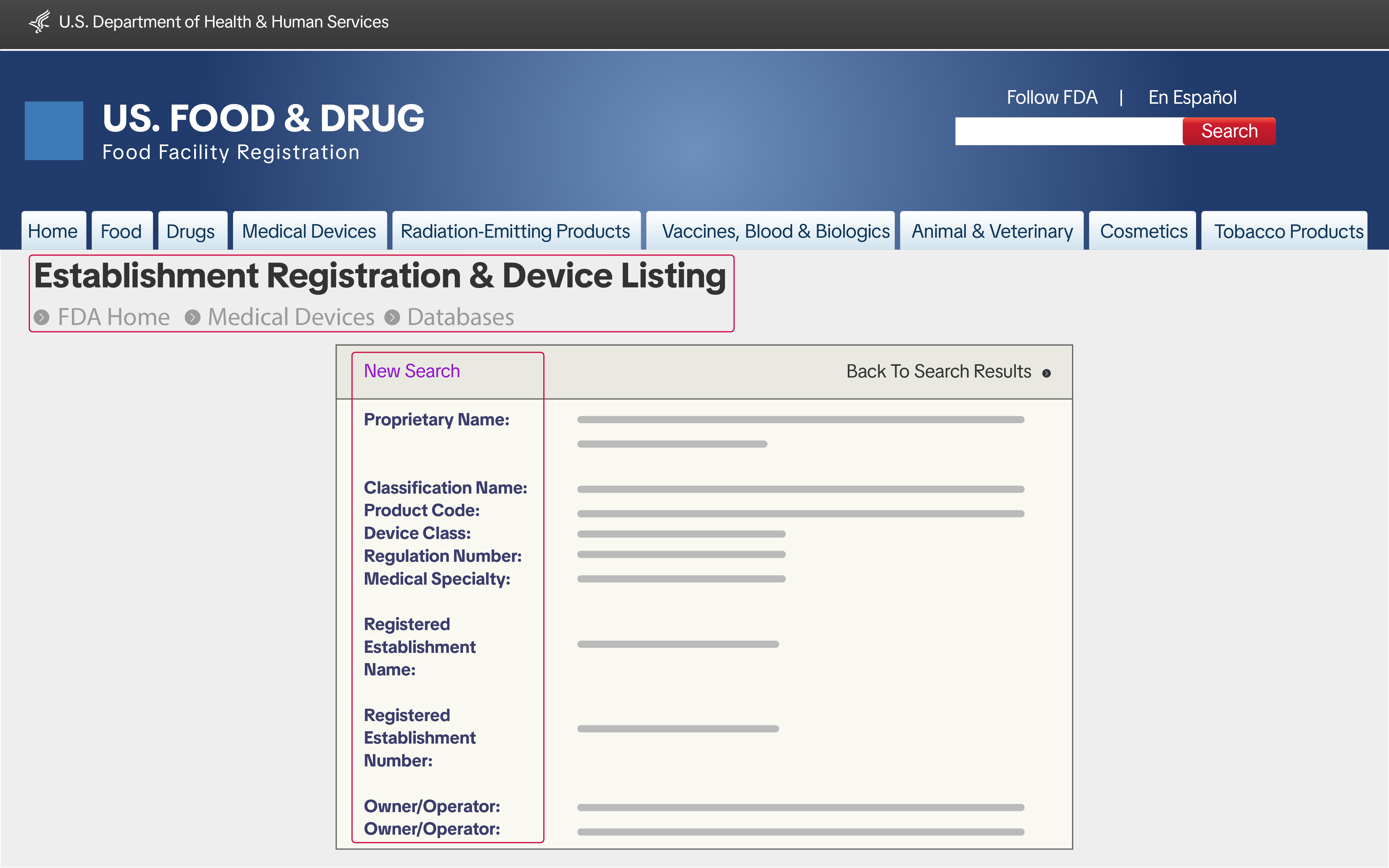 Manufacturers, importers, repackers of Class I and Class II devices must submit a screenshot of their Establishment Registration & Device Listing from the FDA's website and meet the following requirements:
Manufacturers, importers, repackers of Class I and Class II devices must submit a screenshot of their Establishment Registration & Device Listing from the FDA's website and meet the following requirements:- Ensure the Classification Name matches the relevant product category.
Product Photos

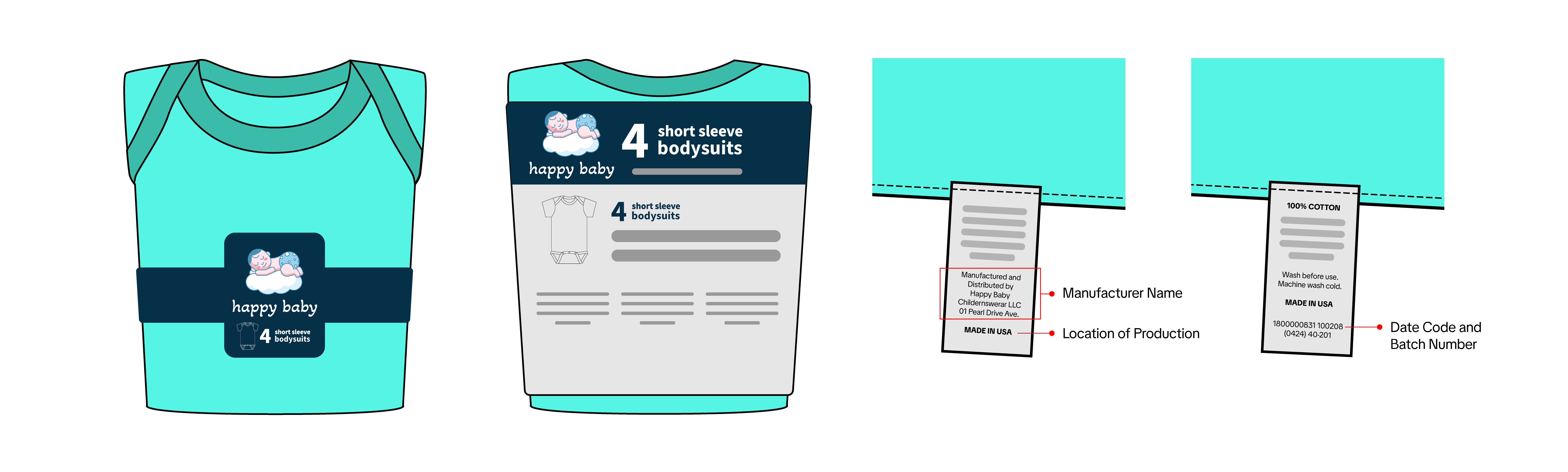 Manufacturers, importers, repackers, and resellers must submit photos of a sample product that meet the following requirements:
Manufacturers, importers, repackers, and resellers must submit photos of a sample product that meet the following requirements:- Show all sides of the product.
- All packaging information must be visible, including product descriptions, warnings, and other relevant details.
- A clear and legible image of the tracking label. Refer to the CPSC’s page on Tracking Labels for more information, including examples and tutorial videos. The tracking label must be securely attached to both the product and its packaging and meet the following requirements:
- Manufacturer/importer or private labeler name
- Location and date of production
- Detailed information on the manufacturing process (e.g., batch number or run number) or other identifying characteristics
- Additional information that specifies the product's source
- Must be in English
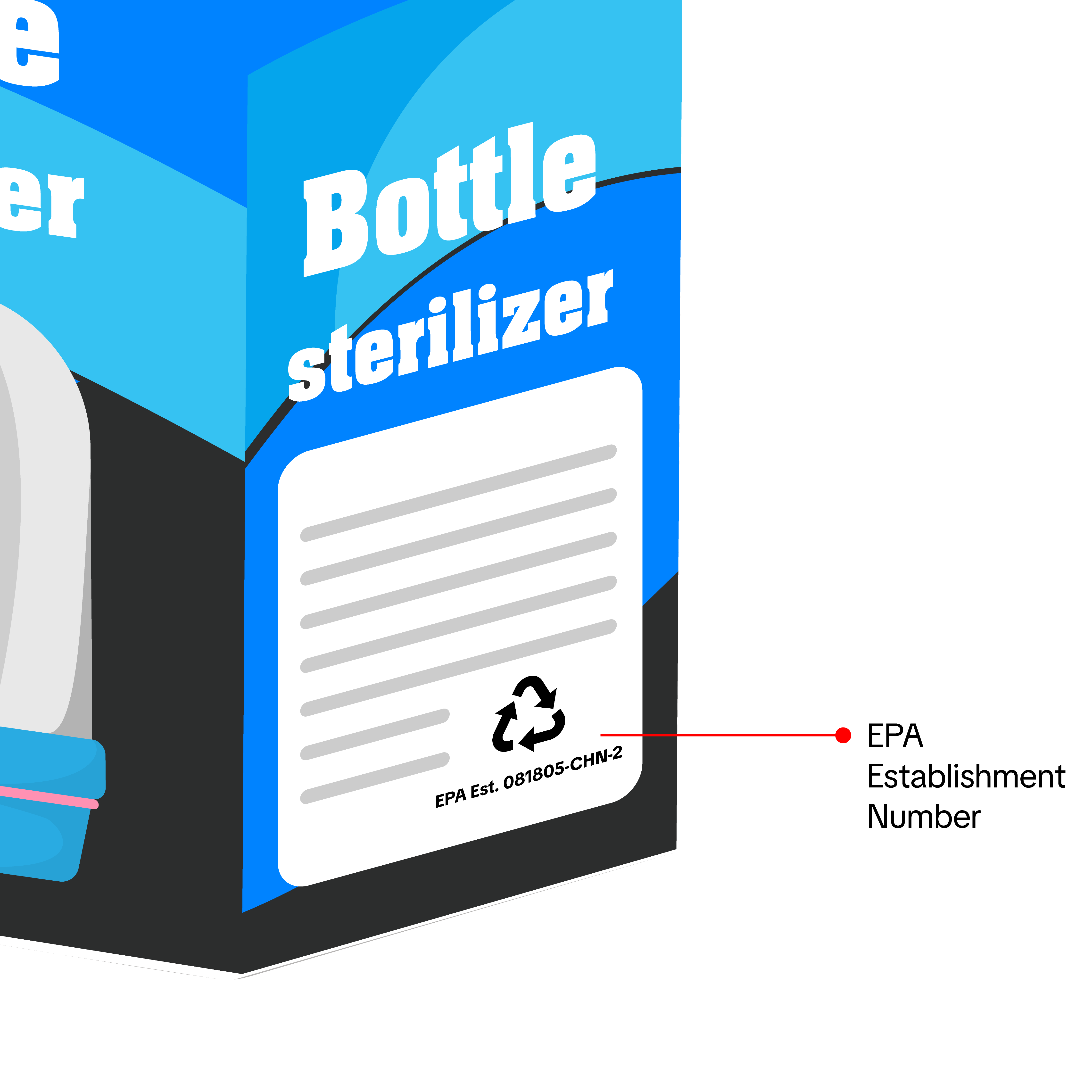 For baby and maternity sterilizers, manufacturers, importers, repackers, and resellers must submit photos of a sample product that meet the following requirements:
For baby and maternity sterilizers, manufacturers, importers, repackers, and resellers must submit photos of a sample product that meet the following requirements:- Show all sides of the product.
- Sterilizers or products with sterilization claims must have an EPA Establishment Number on the label or packaging.
- Products without sterilization claims will not need an EPA Establishment Number (e.g., bottle warmers).
Purchase Invoice
 Resellers must provide a purchase invoice to confirm the authenticity and source of their products. When submitting the purchase invoice, make sure it is legible, free from alterations, and meets the following requirements:
Resellers must provide a purchase invoice to confirm the authenticity and source of their products. When submitting the purchase invoice, make sure it is legible, free from alterations, and meets the following requirements:- Include the supplier’s name and address
- Issued within the last 365 days.
- Product details and quantity must match what you are applying to sell.
- The purchase invoice should be in English.
Test Report issued by a CPSC Accredited Laboratory
 Manufacturers, importers, and repackers for baby and maternity products and medical devices must submit a product test report issued by a Consumer Product Safety Commission (CPSC)-accredited laboratory. Click here for a list of CPSC-approved laboratories.
Manufacturers, importers, and repackers for baby and maternity products and medical devices must submit a product test report issued by a Consumer Product Safety Commission (CPSC)-accredited laboratory. Click here for a list of CPSC-approved laboratories.When submitting your test report, make sure it is legible, free from alterations, and meets the following requirements:
- Include the manufacturer or importer’s name and address.
- Issued by a CPSC-accredited laboratory.
- Issued within the last 365 days.
- Product details should match the product category and photos.
- Include a clear description of the tested product.
- Include the name and address of the CPSC-accredited laboratory.
- Clearly lists all safety standards tested with pass or fail results.
- The test report should be in English.
510(k) Pre-Market Notification or Proof of Exemption
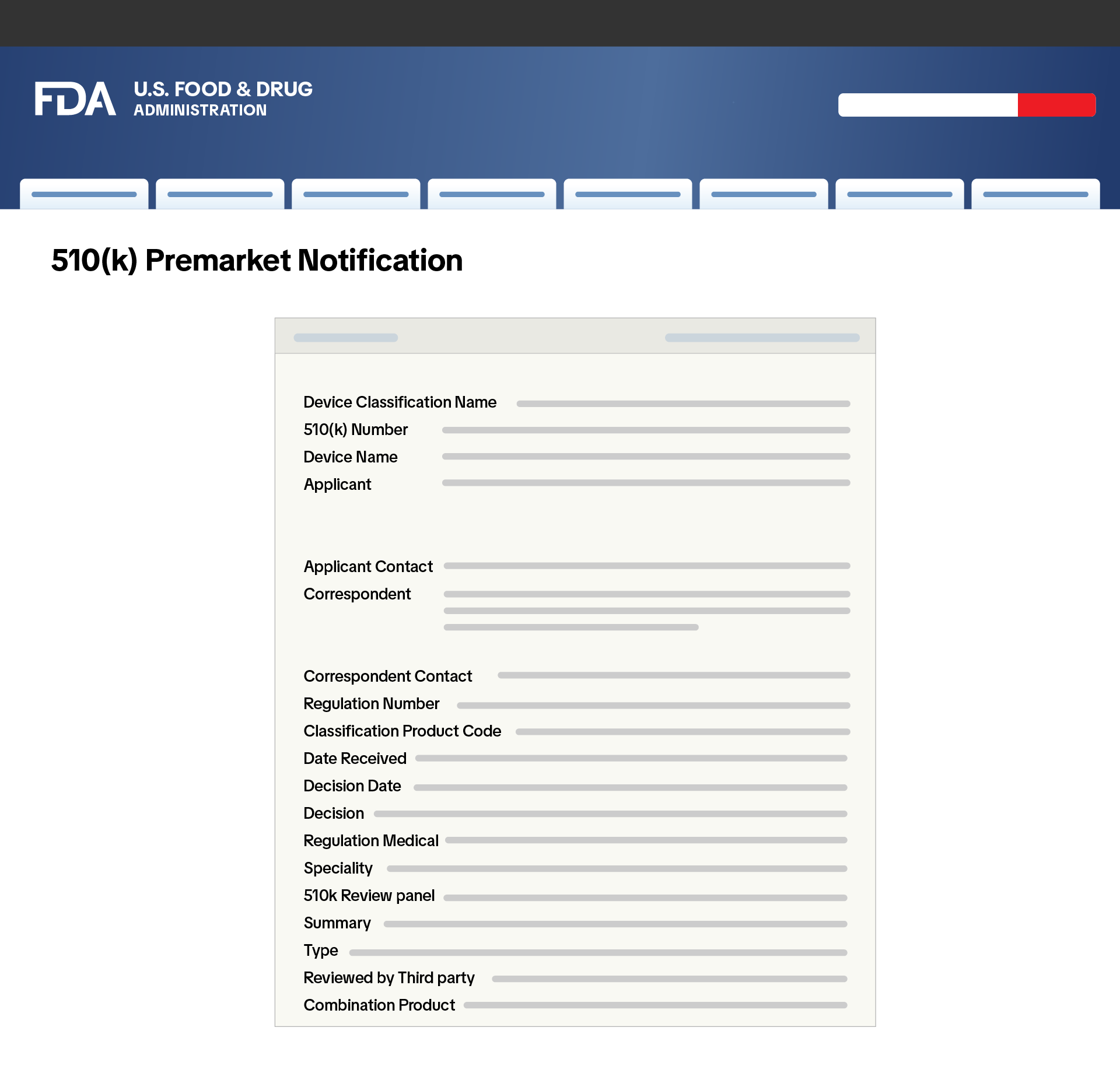 Manufacturers, importers, repackers, and resellers of Class II medical devices must provide either a 510(k) Pre-Market Notification or proof of exemption and meet the following requirements:
Manufacturers, importers, repackers, and resellers of Class II medical devices must provide either a 510(k) Pre-Market Notification or proof of exemption and meet the following requirements:- A screenshot of the 510(k) Pre-Market Notification from the FDA’s website, showing the device is cleared for market.
- Make sure the Device Name listed matches the applicable product category.
- The proof of exemption can be a letter or email from the FDA confirming the device is exempt from 510(k) Pre-Market Notification.
Prohibited Products
The following baby and maternity products are prohibited for sale on TikTok Shop:- Breast pumps and related specialized maternity and nursing equipment
- Vitamins and supplements for children under 3 years of age
- All beaded teethers, including beaded amber teething jewelry, as well as those made from materials like amber, wood, silicone, or other elements
- Potentially dangerous baby products such as weighted baby swaddles and blankets, drop side cribs, co sleepers, crib bumpers etc.
- Products requiring a prescription written by a healthcare provider
- Pregnancy tests
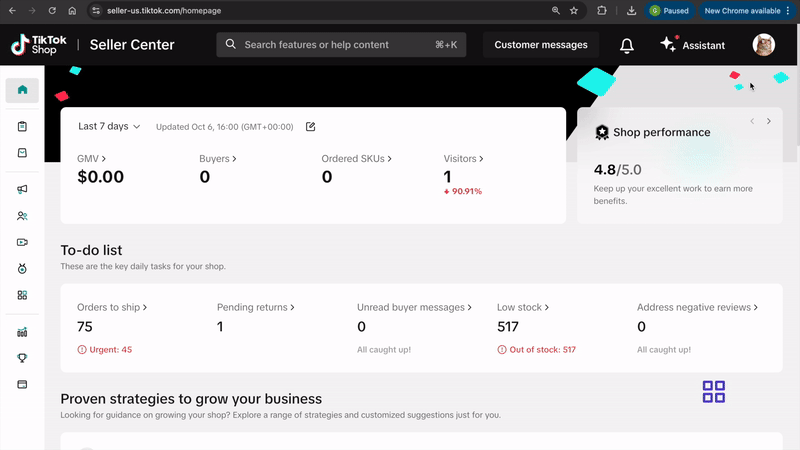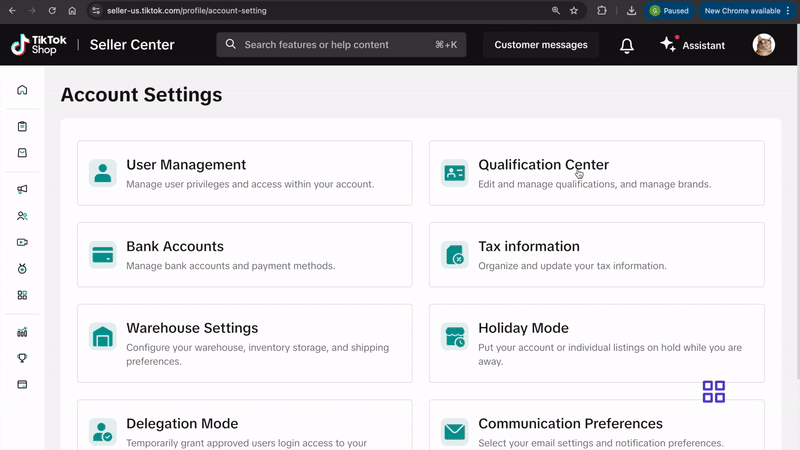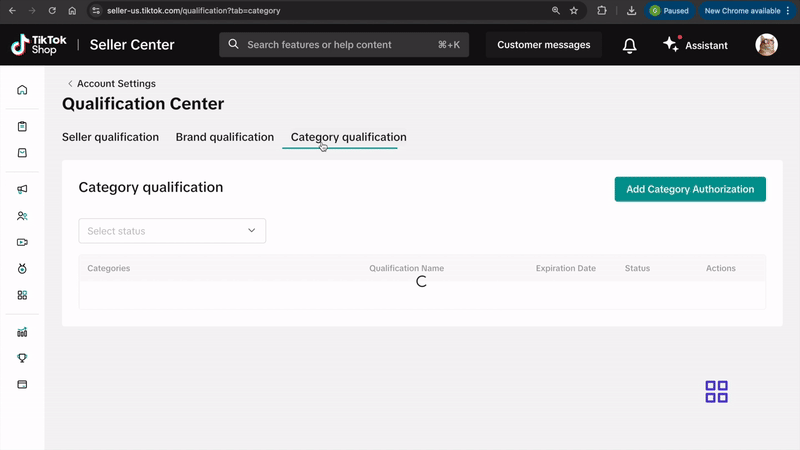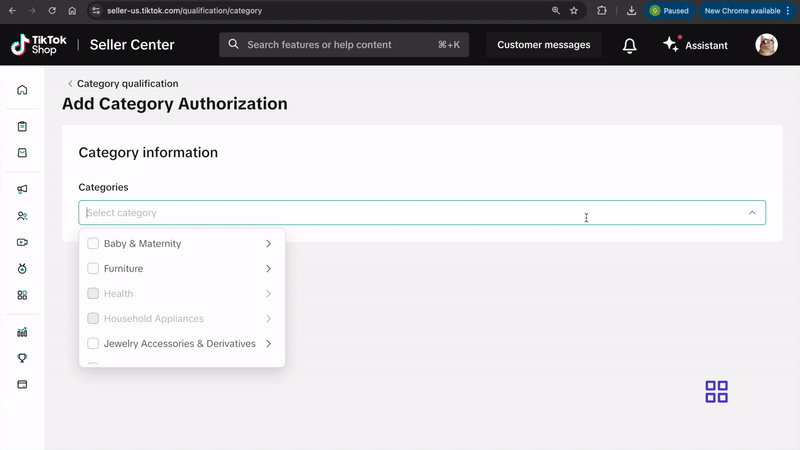
How to Submit Your Documentation
Submit the required documentation through Qualification Center in Seller Center by clicking here or following the steps below.Desktop:
- Log into your TikTok Seller Center account.
- Click on your shop icon at the top right side of the screen.
- Go to My Account > Account Settings.
- Select Qualification Center.
- Click on Category Qualification.
- Click on Add Category Authorization and follow the prompts to submit your application.
Products that claim to be safe for consumer use but are not verified by US FDA, or labeled inaccurately or fraudulently violate the TikTok Shop Restricted Products Policy.